LINK Embrace

www.linkembrace.com
- Plattformsystem
- Konvertierbarkeit
- Unkomplizierte Arbeitsabläufe durch rationelles Instrumentarium
- Prä-OP Planung in 2D & 3D
LINK Embrace Shoulder System
Many years of experience with successful implant systems and fixation concepts, as well as the latest material and coating technologies have been taken into account and applied in the design of the LINK Embrace Shoulder System. Designed as a versatile system, it covers a broad range of indications for reverse treatment in both primary and revision cases.1
System Overview Humeral Options
The LINK Embrace Shoulder System offers diverse fixation options suitable for the majority of patient populations and indications. A wide range of stems in different configurations allows for cemented and cementless fixation. Different types of Reverse Trays and Reverse Inserts offer several options for component alignment in all spatial planes.
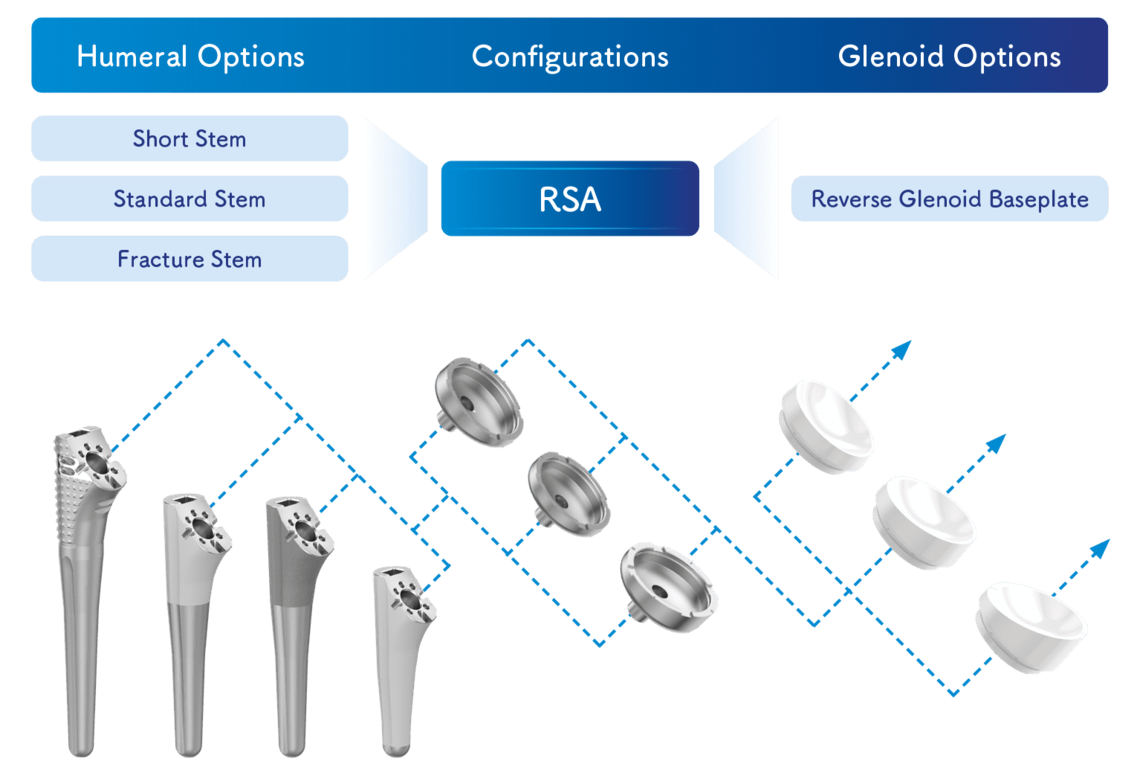
System Overview Glenoidal Options
The Reverse Glenoid Baseplates were designed specifically to address various treatment options depending on surgeon’s choice.1
Reverse Glenoid Baseplate
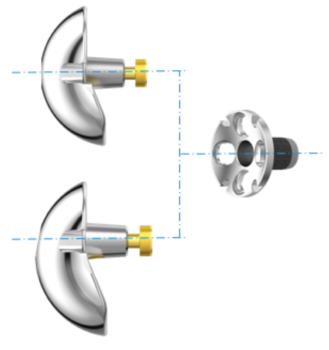
The surgeon can choose multiple screw configurations for the Reverse Glenoid Baseplate. Thanks to its elaborated design it can host a central Bone Screw and up to 4 peripheral Bone Screws. The surgeon can select from angle-stable (locking), polyaxial angle-stable, and standard screw fixation with cortical and cancellous thread design.1 All glenoid implants are designed with a curved back side resulting in bone sparing preparation and better fixation.2 The long-pegged Baseplate is suitable for glenoid reconstruction with grafting to address glenoid defects and glenoid bone loss.3 Glenospheres come in three different diameters, each in neutral and eccentric version.

One of LINK’s major objectives in joint arthroplasty is the preservation of vital bone. In the LINK Embrace Shoulder System, Humeral Short Stems help to save valuable bone stock for possible future treatments and revisions.


For fracture and revision treatment, the system offers a range of Humeral Fracture Stems. These monoblock stems come in several sizes and have a spiked proximal surface. Together with M-l and a-p holes for suture fixation, they allow for stable and physiologic tuberosity reattachment.
The LINK Embrace Shoulder System is implanted by means of a streamlined, lightweight and ergonomic instrument set, allowing for straightforward workflow and high reproducibility. Multi-purpose instruments and elaborated trial components help to reduce the amount of instruments and number of trays, thus enhancing the intra-operative handling and safety for all OR staff, surgeon, and patient.1
- Internal document W. Link DOC-11035
- Strauss et al. The glenoid in shoulder arthroplasty. Journal of Shoulder and Elbow Surgery (2009) 18, 819-833
- Patel M, Rao A, Edirisinghe Y. Glenoid reconstruction for primary or revision shoulder arthroplasty using a metal-backed long pegged glenoid implant and iliac crest autograft or allograft. In Proceedings of the 12th International Congress of Shoulder and Elbow Surgery (ICSES); 2013 Apr 10–12; Nagoya, Japan.