TrabecuLink Augments

The latest material and fixation technologies have been taken into account and used in the design of the TrabecuLink Augments.
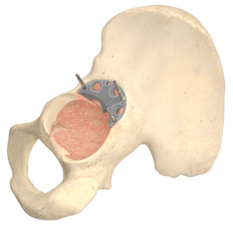
The TrabecuLink Augments offer an attractive solution in cases of segmental acetabular defects as a prosthetic alternative to structural allograft. The biocompatible material Tilastan-E1,2 and the TrabecuLink structure offer an excellent prerequisite for a stable and permanent treatment of bone defects.
Furthermore, the 3-dimensional TrabecuLink structure, with its pore size, porosity and structure depth, also provides an excellent basis for promoting osteoconduction and microvascularization, taking into account the requirements for the structure-covering protein layer (fibronectin - vitronectin - fibrinogen) 3,4.
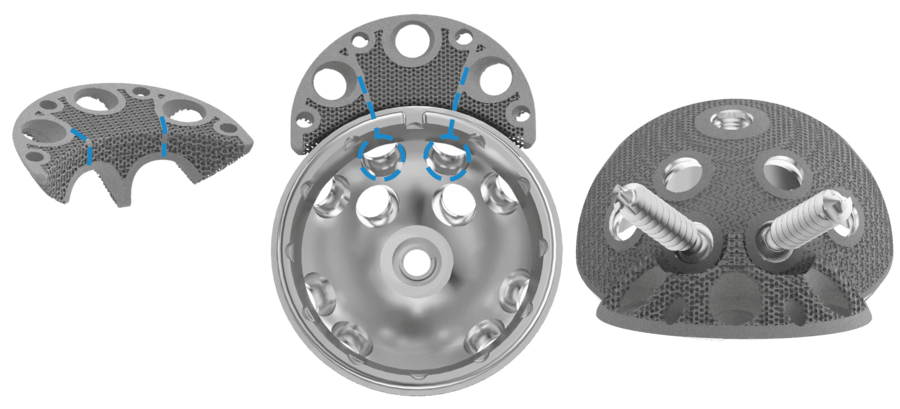
The Augments can be combined with all Link cups, in particular with the MobileLink cup, which has variable options for placing bone screws, as the Augment design allows flexibility to put bone screws through shell and Augment.
In case of acetabular defects the combination of a LINK shell with TrabecuLink Augments can be the solution to help conserve the natural patient anatomic and kinematics.
The Augment size range allows good fit for different anatomies and defects5.
Versatility
- The Augment size range allows good fit for different anatomies and defects5
- Variable bone screw angulation/options
- The Augment design allows flexibility to put bone screws through shell and Augment – big recesses allow for great variability in positing the Augment5
- Low Augment profile
- Augments can be used upside down as buttresses5
Effective fixation
- Sufficient grip of Augments. Augments have a great primary stability5
- Pore geometry of the TrabecuLink structure for effective cell ongrowth3,4,7
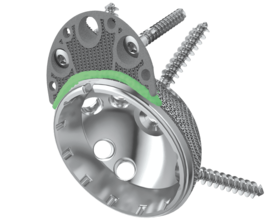
- Great connection between shell and Augment by application of cement mantle (according to surgical technique) 5,6
Reproducible surgical technique
- The Augment forceps helps to place the Augment5
- Temporary fixation of trial Augment and final Augment by drill pins through pin holes - pins which hold trial Augments in place help to position final implant5
- Small inventory
TrabecuLink
3-dimensional structure – for optimal bone ongrowth
- Pore geometry (porosity: 70%, pore size: 610-820 μm, structure depth: up to 1 mm) ensures excellent cell ongrowth 3,4,7
Pore filling
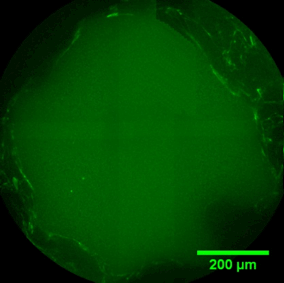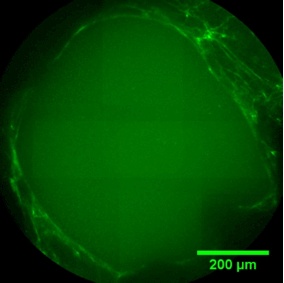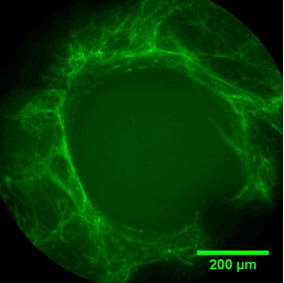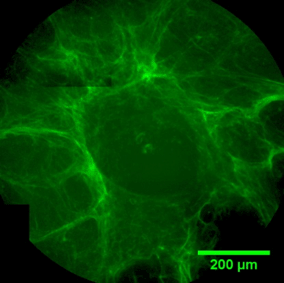
The sequence of images shows a pore of the TrabecuLink structure being filled with tissue under in-vitro cell culture conditions. The fibronectin laid down by human fibroblasts and continually reorganized over a period of eight days is visible as green fibers. Fibronectin is a component of the extracellular matrix that is formed at an early stage of the healing process. It forms a basis for the embedding of collagen, which is essential for mineralization of the tissue and ingrowth of bone into the structure. Apart from the accumulation of fibronectin, which increases over time, a clear contraction of the matrix towards the center of the pore can be observed. This contraction mechanism, which is attributable to the cellular forces acting in the tissue, accelerates the rate at which the pore is filled with tissue, compared to a layer-by-layer tissue growth (Reference: Joly P et al., PLOS One 2013; https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0073545). Julius Wolff Institute, Charité - Universitätsmedizin Berlin
- Data on File, Waldemar Link.
- Wang, Hong, et al. "A comparison of biocompatibility of a titanium alloy fabricated by electron beam melting and selective laser melting." PloS one 11.7 (2016): e0158513
- Cecile M. Bidan, Krishna P. Kommareddy, Monika Rumpler, Philip Kollmannsberger, Yves J.M. Brechet, Peter Fratzl, John W.C. Dunlop et al. (2012) How Linear Tension Converts to Curvature: Geometric Control of Bone Tissue Growth. PLoS ONE 7(5): e36336
- Pascal Joly, Georg N. Duda, Martin Schöne, Petra B. Welzel, Uwe Freudenberg, Carsten Werner, Ansgar Petersen et al. (2013) Geometry-Driven Cell Organization Determines Tissue Growths in Scaffold Pores: Consequences for Fibronectin Organization. PLoS ONE 8(9): e73545. doi.org/10.1371/journal.pone.0073545
- Internal Document, Waldemar Link.
- Beckmann, N. A., et al. "Comparison of the stability of three fixation techniques between porous metal acetabular components and augments." Bone & Joint Research 7.4 (2018): 282-288.
- Steinemann SG: Compatibility of Titanium in Soft and Hard Tissue – The Ultimate is Osseointegration; Materials for Medical Engineering, WILEY-VCH, Volume 2, Page 199-203