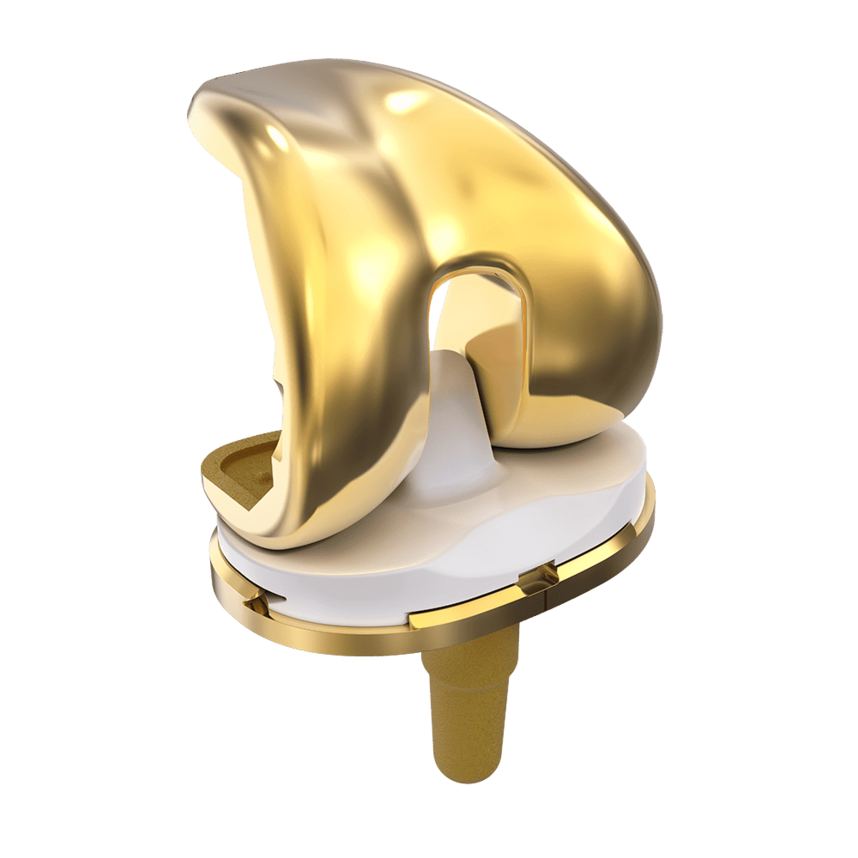
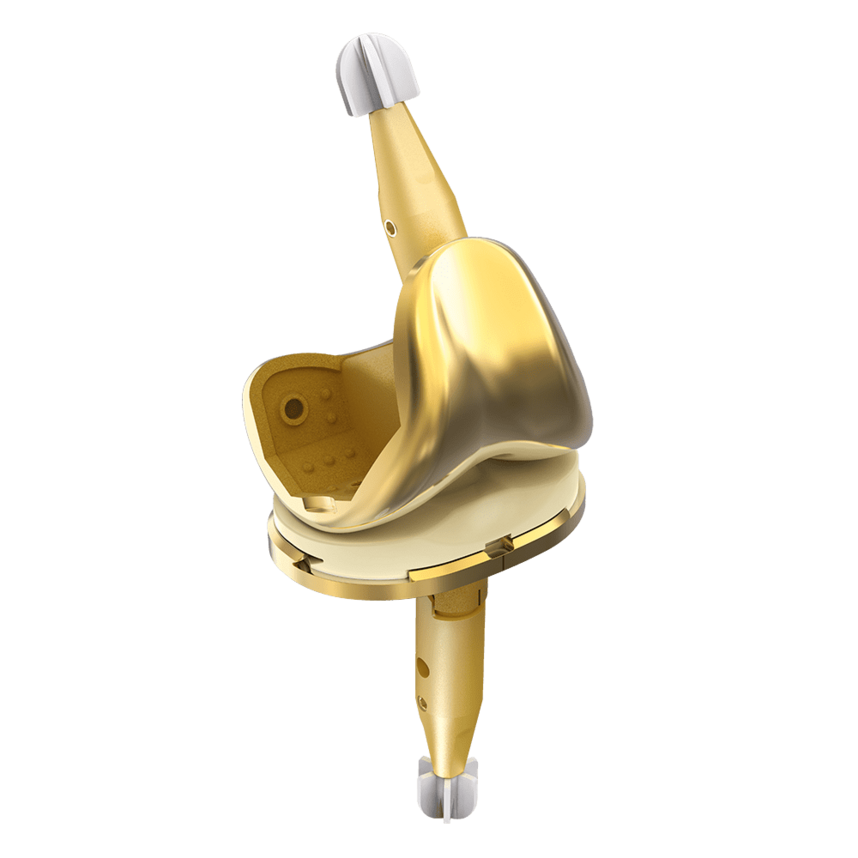
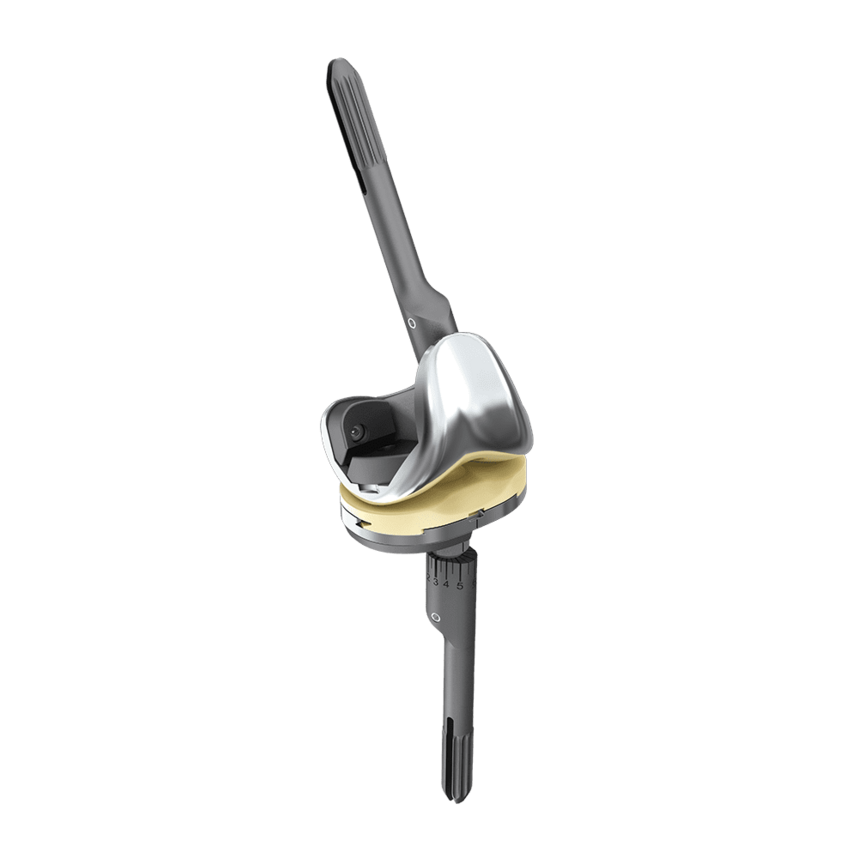
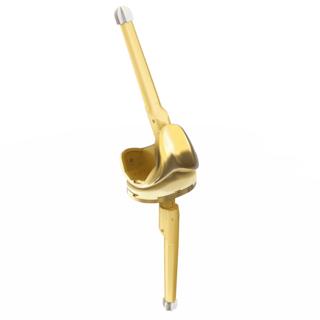
LinkSymphoKnee

Visit our LinkSymphoKnee website: www.linksymphoknee.com
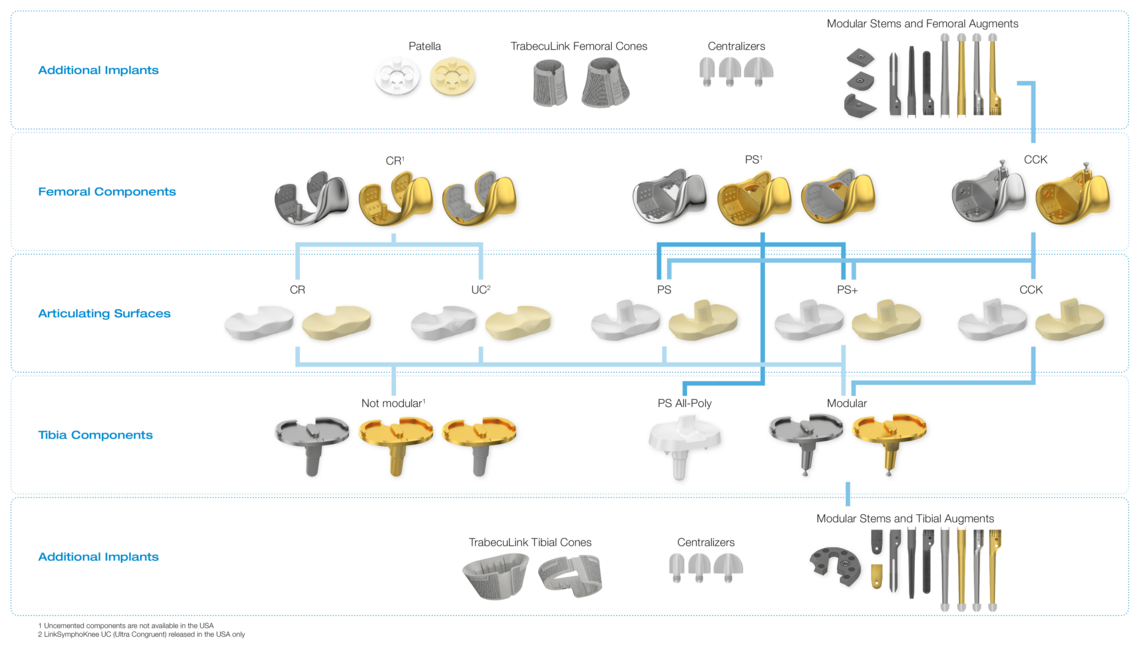
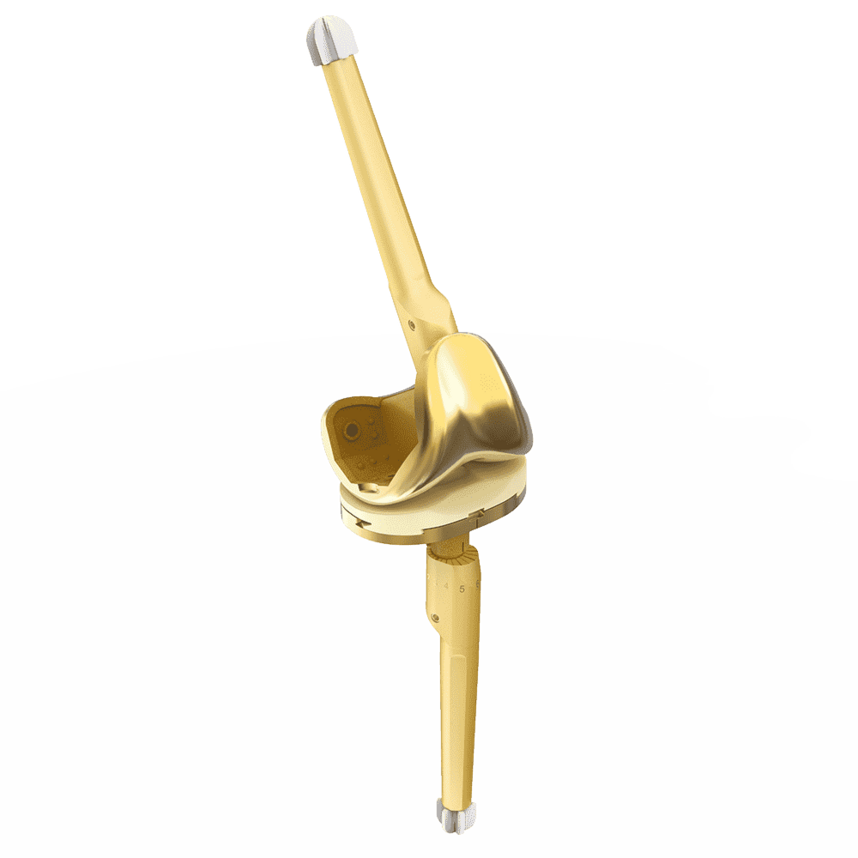
Complete System from Primary to Revision
Wide Range of Sizes
Streamlined Instruments
High-End Materials
- Cobalt Chrome and LINK PorEx1 options
- Wide range of sizes - 14 femoral & 10 tibial
- 2up – 2down femoral tibial size compatibility
- CR, PS, PS+, CCK & All-Poly bearings in UHMWPE & Vitamin E infused cross-linked polyethylene with 1mm increments2
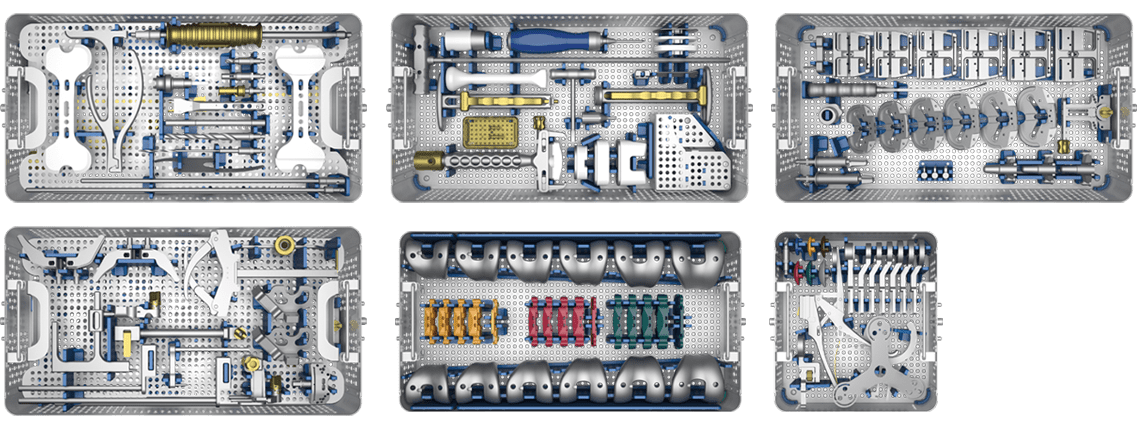
- Elegant, ergonomic instruments configured in 3 trays for a standard CR or PS
- Streamlined instrumentation and optimized implant variability ideal for sameday surgery
Note: Combination with TrabecuLink Femoral and Tibial Cones only in USA cleared.
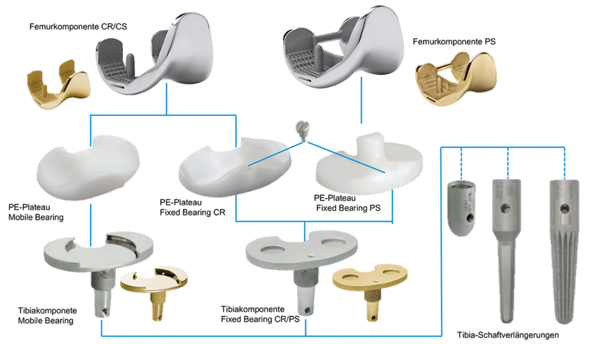
Versions
Fixed Bearing as cruciate ligament retaining (CR), posterior stabilized (PS and PS+) for cruciate ligament sacrificing and revision options (CCK) are available. The high compatibility between the components gives the system a high intraoperative flexibility.
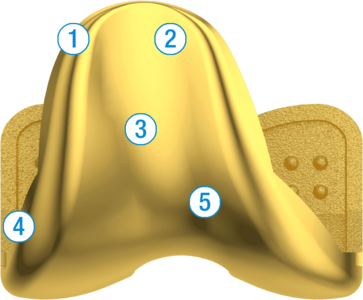
1. Soft Tissue Friendly Anterior Flange
2. Wide Proximal Trochlea
3. Harmonic Growth Between Sizes (3mm)
4. Lateralized Trochlear Groove
5. Anatomical Notch Geometry
1. Bone Conserving Box Geometry
2. Post Engagement at 60° Flexion
3. High PS Cam Location
4. SpheroGrip Macrostructure provides for a homogenous
Bone Cement-Implant Interface
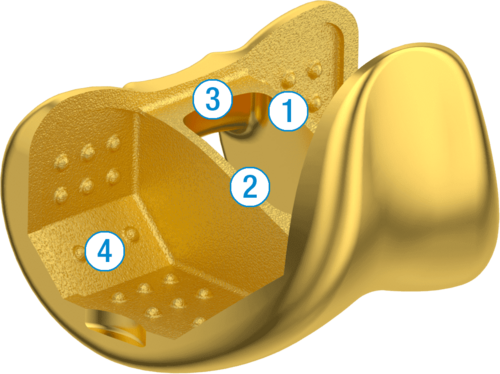
1. 4.5° Anterior Flange
2. Low Profile Patella Flange
3. Gently Decreasing Flexion Radius
4. Graduated Trochlear Groove Depth
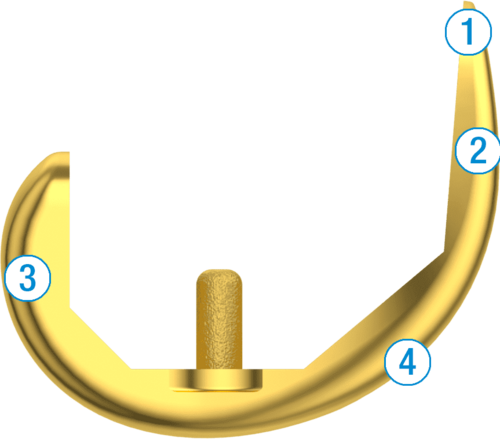
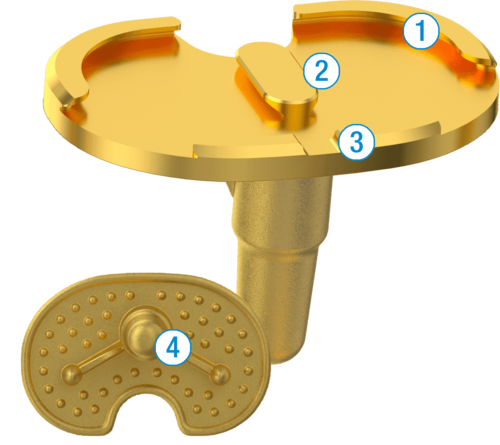
1. Robust Central and Posterior Dovetail Locking Detail
2. Antirotation Island Guides Bearing Implantation
3. Flattened Anterior Lip Designed To Reduce Bearing Insertion Angle
4. Keel and Fin Designed to Protect Against Rotational Forces
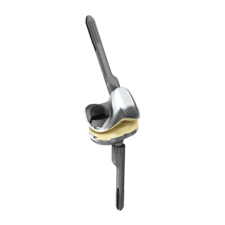
Revision CCK:
- Complete revision system suitable for standard and complex anatomical situations
- Cone friendly femoral and tibial design
- Vast array of straight and offset cemented and uncemented stems
- Precise instrumentation streamlined into four trays
The LinkSymphoKnee System is implanted by means of a streamlined, lightweight and ergonomic instrument set, allowing for straightforward workflow and high reproducibility. Multi-purpose instruments and elaborated trial components help to reduce the amount of instruments and number of trays, thus enhancing the intra-operative handling and flexibility.
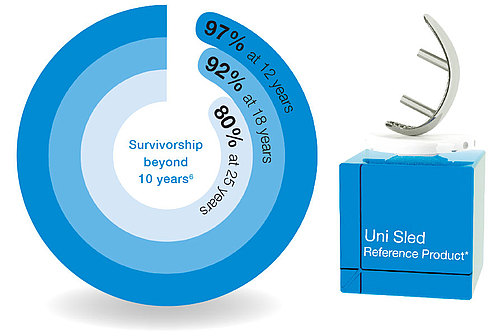
LINK Sled Prosthesis

The LINK Unicondylar Sled Prosthesis was first implanted in 1969. It was last modified in 1981, and ever since the successful implant design has remained unchanged:
Naturally shaped components - Polycentric femur design permits anatomically adapted reconstruction:
- Successful polycentric femur design10, 12
- Bone-conserving design for future arthroplasties14
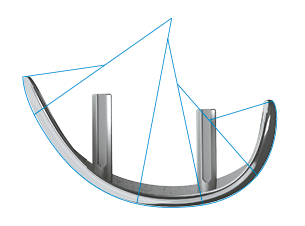
"Round-on-Flat" Design. This design allows excellent freedom of movement, leading to patient specific mobility.13:
- Unrestricted kinematics3, 9, 13
- Symmetrical design allows treatment of medial and also lateral gonarthrosis 2
Proven longevity
- Outstanding clinical outcomes document the device’s reliability11,12
- Extensive clinical experience12
- Unchanged design for three decades
- Excellent documented outcomes12

All-poly design
This version is available in four heights: 7, 9, 11 and 13 mm, and four diameters: 45, 50, 55, and 58 mm.

Metal-backed design
This version is available in four heights: 8, 9, 11, and 13 mm and three diameters: 45, 50, and 55 mm.

LINK PorEx Surface Modification
- Significant reduction of plastic wear and release of metal ions1
- Ceramic-like abrasion behavior1
- Outstanding hardness1


Femoral components
The femoral components are available in four sizes:
• Small (16 x 40 mm)
• Medium-small (17 x 46 mm)
• Medium (18 x 52 mm)
• Large (20 x 60 mm)
In the event of a revision, explantation is easier than with undercut stem structures.14
Tibial plateaus
The symmetrical shape of the tibial plateaus allows them to be used both medially and laterally. The dimensioning is adapted to the shape of the tibial head anatomy.
- Ackroyd, C., 2003. Journal of Bone & Joint surgery BR - Medial compartment arthroplasty of the knee. Bristol: s.n.
- Ashraf, T., Newman, J., Evans, R. & Ackroyd, C., 2002. Journal of Bone & Joint surgery BR - Lateral unicompartmental knee replacement. s.l.:From Southmead Hospital, Bristol, England.
- Dreyer, P. D. J., Späh, D. H. & Teichner, D. A., 1984. Längerfristige Erfahrungen mit Schlittenendoprothesen St. Georg - Zeitschrift für Orthopädie. Bremen: s.n.
- Internal Technical Report, kein Datum Internal technical report: Study of the influence of TiNbN-coating on the ion release of CoCrMo-alloys in SBF buffer simulator testing. s.l.:s.n.
- Mackinnon, J., Young, S. & Baily , R., 1988. The St Georg sledge for unicompartmental replacement of the knee - Journal of Bone&Joint Surgery BR. Bristol: s.n.
- Müller, J., 2016. Link Schlittenprothese [Interview] (Juli 2016).
- Müller, J., 2018. Unicondylar Sleds - Indications & Overview, Suzhou, China: 2018 LINK SLED Summit Symposium.
- Newman, J. & et. al., 2009. Unicompartmental or total knee replacement. The 15-year results of a prospective randomised controlled trial - Journal of Bone & Joint Surgery. s.l.:s.n.
- Nieder, E., 1991. Schlittenprothese, Rotationsknie und Scharnierprothese Modell St. Georg und Endo Modell. Orthopäde, pp. 170-180.
- Steele, R., Evans, R., Achroyd, C. & Newman, J., 2006. Survivorship of the St. Georg medial sled unicompartmental knee replacement beyond ten years. s.l.:s.n.
- Swedish Knee Arthroplasty Register , 2012. Swedish Knee Arthoplasty Register - Annual Report 2012. [Online]
Available at: http://www.myknee.se/pdf/117_SKAR_2012_Engl_1.0.pdf
[Accessed on March 26, 2020]. - Swedish Knee Arthroplasty Register, 2015. Swedish knee arthroplasty register - Annual report 2015. [Online]
Available at: http://www.myknee.se
[Accessed on July 4, 2018]. - Weale, A., Murray, D., Newman, J. & Ackroyd, C., 1999. Journal of bone & joint surgery BR - The length of the patellar tendon after unicompartmental and total knee replacement. Bristol: s.n.
- Wohlgemut, D. G., 2009. Die Funktion des Kniegelenks wiederherstellen - Minimalinvasiver Kniegelenkersatz [Interview] 2009.
- Gleeson, R.E., Evans, R., Ackroyd, C.E., Webb, J., Newman, J.H.: Fixed or Mobile Bearing Unicompartmental Knee Replacement? Comparative Cohort Study, The Knee 11 (2004) 379-384.
- Gleeson, The Swedish Knee Arthroplasty Register, Validity and Outcome, Department of Orthopedics Lund University Hospital, SE-221 85 Lund, Sweden, 2011.
GEMINI SL Total Knee Replacement

- High degree of joint stability and good kinematics
- High primary stability
- Natural joint reconstruction with physiological freedom of movement and functionality1, 2, 3
Successful long-term clinical outcomes4
- 5 yrs 97.5%
- 10 yrs 95.5%
- Extended range of indications and comprehensive treatment options with intraoperative flexibility
Designs
Numerous different versions enable customization to suit different bone structures and possible sensitivity. The system permits cemented and cementless anchorage. Additional PorEx Surface Modification can reduce ion release, and can also reduce wear because the coefficient of friction is lower.14 Modular tibial components allow approximation of tibial stems, which can ensure stability when bone condition is poor.7
Versions
Fixed Bearing as cruciate ligament-conserving (CR) or cruciate ligament replacement (PS) and Mobile Bearing (CR/CS) are available. GEMINI SL Fixed Bearing consists of a single tibial metal casing, which enables both cruciate ligament preservation (CR) and cruciate ligament replacement (PS) options. This simplifies the choice of implant and gives intraoperative flexibility.

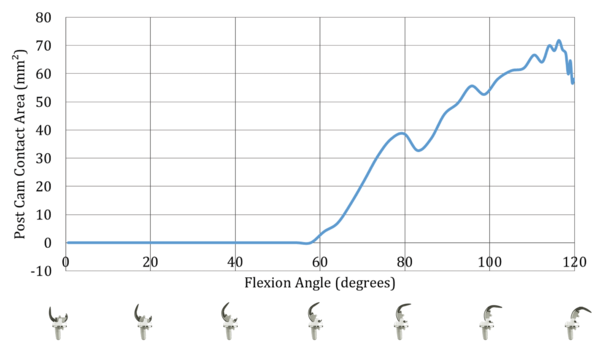
Features of GEMINI® SL® Fixed Bearing PS:
- Post-cam attachment comparable to the natural knee13
- Uniformly large contact surface on the pin
- Low surface load on the pin13
- Reliable and secure coupling mechanism13
- Minimal luxation risk13.
- Enlarged contact surface during flexion with reduced contact load and minimal fracture risk and abrasion.13
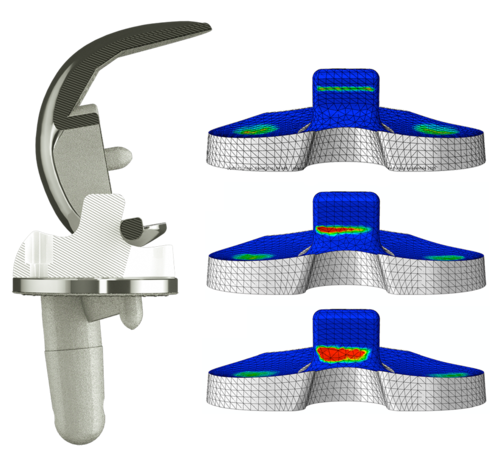
If the ligaments and capsule are intact and joint stability is good, the Fixed Bearing Cruciate Retaining (CR) version can be used for natural joint reconstruction.
The Fixed Bearing CR with moderate congruence reduces the bone loading by means of enhanced load distribution, and enables natural femoral rollback and femur rotation15.
If the posterior cruciate ligament is insufficient or has been removed,
the Fixed Bearing Posterior Stabilized (PS) version is used.

The freedom of rotation provided by GEMINI SL Mobile Bearing helps to preserve the alignment of the patellofemoral and also femorotibial articulations during knee flexion. Self-alignment by means of rotation of the polyethylene PE articulating surfaces improves the postoperative kinematics.11
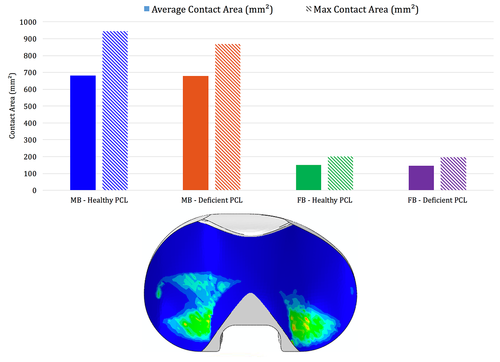
At the same time, the polished tibial base plate improves the abrasion behavior.10, 11
GEMINI SL Mobile Bearing offers the advantage of a highly compliant joint geometry with reduced surface and substrate stress distribution, while the Mobile Bearing articulation reduces the development of boundary surface bone loads.9
Good mobility with an enlarged articulation surface.
The high degree of articulation congruence stabilizes the knee, also in cases of loss of the posterior cruciate ligament.8, 9, 12
In the production of Vit-E plateaus, vitamin E is used as an antioxidant in order to protect the material by neutralizing the free radicals created by highly crosslinking. The product's mechanical properties and biocompatibility are preserved 19,20,21,22,23
Highly Cross-Linked by irradiation
- Improved wear resistance for delamination as well as abrasion – Reduction up to 94%16,17
Vitamin-E-Doped
- Stabilizing of UHMWPE by enrichment with Vitamin E respectively synthetic available product alpha-tocopherol.
Reduction of free radicals improves durability. Embrittlement decreases18
Visit our spar-k microsite for information about the new spar-k instrument set:
spark.linkorthopaedics.com
- H. Thabe, „Auswirkungen verschiedener konstruktiver Prothesenmerkmale auf Langzeitergebnisse“, Akt Rheumatol 2013;38.
- Internal data - H. Thabe, „Aspekte zum Konzept der beweglichen Tibiaplateaukonstruktion, April 2000.
- J. Goodfellow, “The Mechanics of the Knee and Prosthesis Design”. J Bone Joint Surg Br 1978; 60:358-369
- ripo.cineca.it/pdf/relazione_2016_v19_inglese.pdf
- Internal data - H. Thabe, “ Arthroprometic sizing in TKA / GEMINI MK2”
- P.S. Walker, “A Comparative Study of Uncemented Tibial Components”. J Arthroplasty 1990; 5:245-253
- A. Completo et al., “The influence of different tibial stem designs in load sharing and stability at the cement-bone interface in revision TKA”. Knee 2008;15:227-232
- S. Bignozzi, “Three different cruciate-sacrificing TKA designs: minor intraoperative kinematic differences and negligible clinical differences”. Knee Surg Sports Traumatol Arthrosc 2014; 22:3113-3120
- B. Innocenti, GEMINI SL Mobile Bearing / Fixed Bearing CR Biomechanical analysis in healthy and deficient PCL patient LINK 999_WP_003_2017_Gemini-SL_en, 2017
- J. Callaghan, “Mobile-Bearing Knee Replacement: Concept and Results”. AAOS Instructional Course Lectures 2001; 50:431-449
- D. Dennis, “Mobile Bearing Total Knee Arthroplasty Design Factors in Minimizing Wear”. Clin Orthop Relat Res. 2006; 452:70-77
- Internal data - S. Greenwald, “Classification of Mobile Bearing Knee Design: Mobility and Constraint”, 2002
- B. Innocenti, GEMINI SL Fixed Bearing PS: Biomechanical Analysis of the Post-Cam System. LINK 999_WP_002_2017_Gemini-SL_en, 2019
- Internal technical report: Study of the influence of TiNbN-coating on the ion release of CrCrMo-alloys in SBF buffer simulator testing.
- GR Scuderi, WN Scott, “Total Knee Arthroplasty. What we have learned.”1996; Am J Knee Surg 9:73-75
- S. M. Kurtz, „The Origins and Adaptations of UHMWPE for Knee Replacement“, in UHMWPE Biomaterials Handbook, S. M. Kurtz, Ed., Burlington, MA Academic Press 2009.
- S. M. Kurtz, „Advances in the Processing, Sterilization, and Crosslinking of Ultra-high Molecular Weight Polyethylene for Total Joint Arthroplasty“, Biomaterials 1999; 20:1659-1687.
- E. M. Brach del Prever, „UHMWPE for Arthroplasty: Past or Future?“, J Orthopaed Traumatol 2009; 10:1-8
- Produktakte W. LINK (Quadrant, MediTECH Data Sheet)
- E. Oral, „Characterization of Irradiated Blends of Alpha-tocopherol and UHMWPE“, Biomaterials 2005; 26(33):6657-6663.
- E. Oral, „Highly Crosslinked UHMWPE Doped with Vitamin E“, in UHMWPE Biomaterials Handbook, S. M. Kurtz, Ed., Burlington, MA Academic Press 2009.
- S. M. Kurtz, „Vitamin-E-Blended UHMWPE Biomaterials“, in UHMWPE Biomaterials Handbook, S. M. Kurtz, Ed., Burlington, MA Academic Press 2009.
- S. M. Kurtz, „Trace Concentration of Vitamin E Protect Radiation Crosslinked UHMWPE from Oxidative Degradation“, J Biomed Mater Res A 2008; 549-563
- B. Innocenti, Biomechanical analysis of GEMINI SL total knee replacement implant designs up to 155° of flexion. LINK 999_WP_001_2017_Gemini-SL_en, 2019
LINK Endo-Model

The LINK Endo-Model has been in use for over 30 years as a rotational or hinge knee prosthesis for both primary and revision arthroplasties. The Endo-Model Rotational Knee Prosthesis gives you a multiplicity of options.
The method for implanting the Endo-Model is simple and uncomplicated.2, 6 The prosthesis has an intracondylar and an intramedullary fixation, which provide a high degree of stability and protection against bacteria.1, 7 For patients with a hypersensitivity to metal, LINK also offers a special surface coating (LINK PorEx).13
The Endo-Model is an implant with intrinsic stability.1, 5, 12 The various options (Endo-Model Standard, Endo-Model Modular, Endo-Model SL, Endo-Model W) offer a high degree of flexibility.
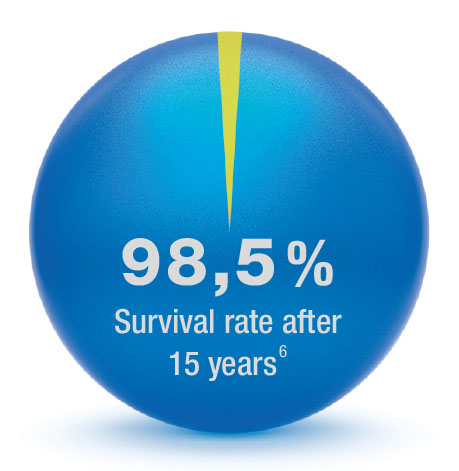
The Endo-Model has been used successfully for over 30 years now, and can boast an unparalleled clinical history with excellent clinical results.1, 2, 5, 8 Even after 15 years, the Endo-Model has a survival rate of 98.5%.9
The Endo-Model enables speedy and uncomplicated implantation, while the design of the implant ensures good postoperative function.10, 11 Its excellent kinematics mean that the Endo-Model gives a high level of stability, especially in extension.3, 11 The implant allows flexion of the joint up to 142° and hyperextension of 2°.
Sizes:
4 different sizes for femoral and tibial components, for right and left in each case (XS, S, M, L).
Material:
CoCrMo, UHMWPE and LINK PorEx
Mechanism:
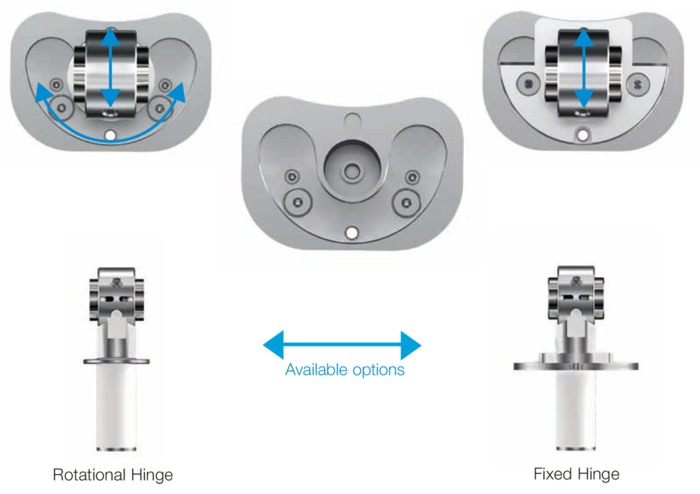
Available in rotational and hinge versions.
Type of fixation:
Cemented
Centralizers:
The shape of the centralizers enables a central position in the medullary canal. Furthermore, the centralizers prevent contact between the metal stem and the cortical bone, thus reducing stress peaks in the bone when bending forces are acting.3
LINK PorEx Technology:
For metal-sensitive patients, LINK offers a surface modification.13
The modular intracondylar total knee prosthesis, Endo-Model – M, is an addition to the Endo-Model Standard Knee Prosthesis.
Sizes:
4 different sizes for femoral and tibial components, for right and left in each case (XS, S, M, L).
Stem sizes:
Modular stem, cemented (EndoDur-S, LINK PorEx*): 50 mm-280 mm
Modular stem, cementless, tapered (Tilastan-S): 50 mm-280 mm
Modular stem, cementless, cylindrical (Tilastan-S): 60 mm (Ø 10-18 mm), 120 mm-280 mm (each size available in the following diameters: Ø 12-18 mm)
Mechanism:
Available in rotational and hinge versions.
Type of fixation:
Cemented and cementless
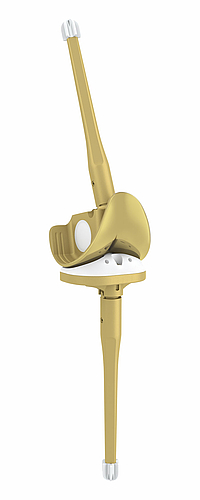
Centralizers:
The shape of the centralizers enables a central position in the medullary canal. Furthermore, the centralizers prevent contact between the metal stem and the cortical bone, thus also preventing stress peaks in the bone when bending forces are acting.3
LINK PorEx Technology:
The implant is also available with a coating for metal-sensitive patients.13
LINK MEGASYSTEM-C - Modular joint pairings, Endo-Model with female taper. Total condyle replacement and intracondylar versions.
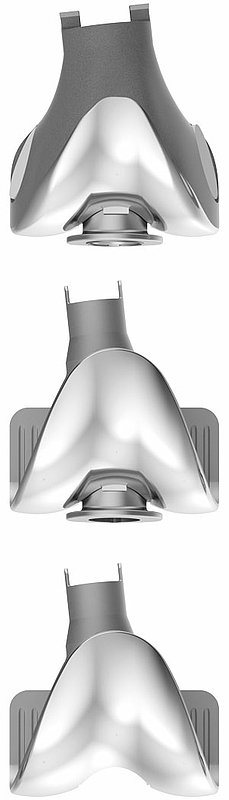
The modular intracondylar total knee prosthesis, Endo-Model – W, is an addition to the Endo-Model Knee Prosthesis.
Sizes:
3 different sizes for femoral and tibial components, for right and left in each case (S, M, L).
Stem sizes:
Modular stem, cemented (CoCrMo): 100 mm-160 mm (Ø 12-16 mm)
Modular stem, cementless (Tilastan): 100 mm-160 mm (Ø 12-24 mm)
Material:
CoCrMo, UHMWPE and LINK PorEx**
Mechanism:
Available in a rotational version.
Type of fixation:
Cemented and cementless
Centralizers:
The shape of the centralizers enables a central position in the medullary canal. Furthermore, the centralizers prevent contact between the metal stem and the cortical bone, thus also preventing stress peaks in the bone when bending forces are acting.3
Compatibility:
The Endo-Model-W is compatible with the MEGASYSTEM-C. All the stems from the MEGASYSTEM-C which have a male taper can be used.
The high degree of modularity of this system allows both, partial bone replacement in the proximal and distal femur regions in small increments and also total femur replacement.
You can find further information under MEGASYSTEM-C.
*Internal designation
**Only custom-made
Images from above:
- LINK Endo-Model EVO – W Modular Joint Component, Condylar Replacement, Rotating Hinge
- LINK MEGASYSTEM-C Endo-Model female taper, Intracondylar Version, Rotating Hinge
- LINK MEGASYSTEM-C Endo-Model female taper, Intracondylar Version, Pure Hinge
- Petrou et al., Medium-term results with primary cemented rotating-hinge total knee replacement - A 7- to 15- year follow-up, THE JOURNAL OF BONE & JOINT SURGERY (Br), 2004
- Nieder et al., Mid-term results of 1837 cases at Primary Knee Arthroplasty Follow-up period 2-12 years (mean 6.6 years), Scientific Exhibition: 20th SCIOT World Congress, 1996
- Engelbrecht et al., Die Rotationsendoprothese des Kniegelenks, Springer Verlag,1984
- Bistolfi et al., Endo-Model® Rotating-hinge Total Knee for Revision Total Knee Arthroplasty, Orthopedics, 2013
- Sanguineti et al., Total knee arthroplasty with rotating-hinge Endo-Model® prosthesis: clinical results in complex primary and revision surgery, Arch Orthop Trauma Surg, 2014
- Bistolfi et al., Results with the Endomodell rotating hinged knee prosthesis after 18 years of follow-up, University of Turin: Department of Orthopedics and Traumatology
- Lazano, Better Outcomes in Severe and Morbid Obese Patients (BMI>35kg/m^2) in Primary Endo-Model® Rotating-Hinge Total Knee Arthroplasty, The Scientific WorldJOURNAL, 2012
- Dae Kyung Bae et al, Long-Term Outcome of Total Knee Arthroplasty in Charcot Joint: A 10- to 22-Year Follow-u, THE JOURNAL OF Arthroplasty, 2009
- Mavrodontidis et al., Application of the Endomodel Rotating Hinge Knee Prosthesis for Knee Osteoarthritis, Journal of surgical orthopaedic advances, 2008
- Argenson et al., Total knee arthroplasty in femorotibial instability, Orthopäde S45-47, 2000
- Atrey et al, A 3 year minimum follow up of Endoprosthetic replacement for distal femoral fractures - An alternative treatment option, Journal of Orthopaedics,2017
- Felli et al., The Endo-Model® rotating hinge for rheumatoid knees, Orthopäde, 2016.
- Bader et al. Alternative Werkstoffe und Lösungen in der Knieendoprothetik für Patienten mit Metallallergie, Der Orthopäde 2008
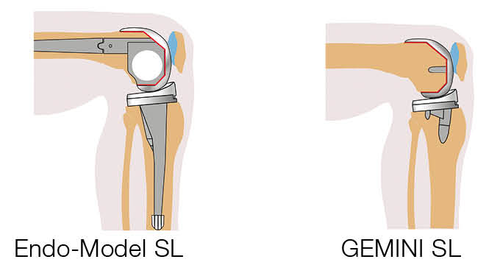
LINK Endo-Model SL

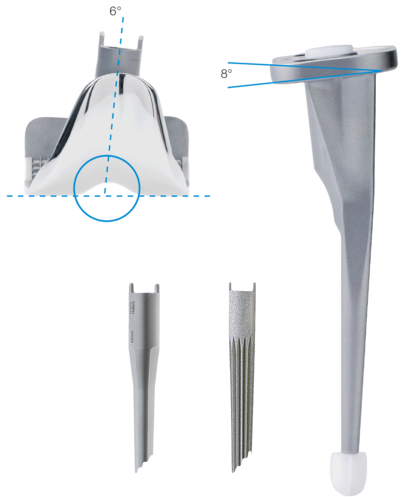
Anatomically Adapted
- 6° valgus from the joint line
- Tibial plateau with 8° slope to dorsal
- The deep patellar articulating groove creates physiological patellar movement and patellar self-guidance2
Wide Range of Stems
- Modular stems, cemented and cementless, for femur and tibia
- Cemented monoblok stem for tibia
Reproducible Surgical Technique
- Interfaces, tailored to the
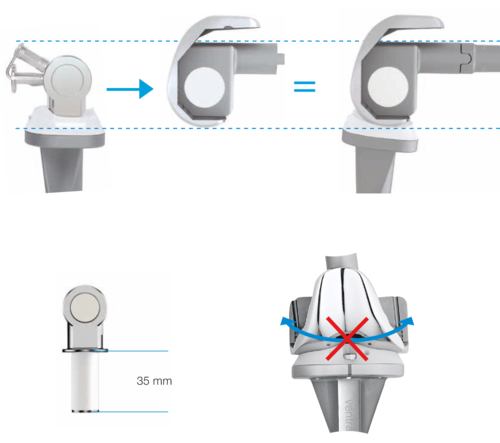
LINK GEMINI SL Primary Knee - Modern, modular instrument set
Flexible
- Intraoperative changeover from rotational to hinge knee with implant components in situ
- Intraoperative flexibility because fully compatible with MEGASYSTEM-CTumor and Revision System1
Designed for Reliability and Stability
- Based on the LINK Endo-Model
- Large “jump distance” for more secure knee flexion
Coupling and Decoupling in the Joint Plane
- Minimal soft tissue distraction during reduction
Rotational Stability in Extension
- Stability even if there is soft tissue damage
- The implant was developed to achieve a natural gait – combined with maximum reliability
LINK Endo Model SL Rotational and Hinge Knee Prosthesis – fixed hinge version V02
- Rodolfo Capanna MD, Guido Scoccianti MD, Filippo Frenos MD, Antonio Vilardi MD, Giovanni Beltrami MD, Domenico Andrea Campanacci MD, What was the Survival on Megaprostheses in Lower Limb Reconstruction after Tumor Resection, Clin. Orthop. Relat Res. (2015) 473: 820-830
- H. Thabe, „Auswirkungen verschiedener konstruktiver Prothesenmerkmale auf Langzeitergebnisse“, Akt Rheumatol 2013;38
Further Literature
Development based on the Endo-Model® Rotational and Hinge Knee Prosthesis, which has been used with great success for many years.
E. Engelbrecht, A. Siegel, J. Röttger, and Prof. H. W. Buchholz*
Statistics of Total Knee Replacement: Partial and Total Knee Replacement, Design St. Georg
Journal of Clinical Orthopaedics, 1976, No. 120, pp 54-64 (K3)
E. Engelbrecht, E. Nieder, E. Strickle, A. Keller
Intrakondyläre Kniegelenkendoprothese mit Rotationsmöglichkeit
- ENDO-MODELL®
CHIRURG 52: 368-375 (1981) (K1)
R. Dederich und L. Wolf
Kniegelenkprothesen-Nachuntersuchungsergebnisse
Unfallheilkunde (1982) 85:359-368 (K2)
J. Röttger, K. Heinert
Die Knieendoprothesensysteme (Schlitten- und Scharnierprinzip).
Beobachtungen und Ergebnisse nach 10 Jahren Erfahrung mit
über 3700 Operationen.
Z. Orthop. 122(1984) 818-826 (K17)
E. Nieder, E. Engelbrecht, A. Keller
Totale intrakondyläre Scharniergelenkendoprothese mit
Rotationsmöglichkeit - Endo-Modell®
Reprint from Issue 5: Orthopädische Praxis, 1987, 23. Jahresgang,
Seite 402-412 (K34)
K. Heinert, E. Engelbrecht
Total Knee Replacement - Experience with a Surface and
Total Knee Replacement: Further Development of the Model St. Georg®. 2400 Sledges and Hinges
Proceedings of the International Symposium on Total Knee Replacement, May 19-20, 1987, Nagoya, Japan Springer Verlag:, Berlin Heidelberg, New York Tokyo (1987),
pp 257-273 (K53)
E. Engelbrecht, M.D.
The Tibial Rotating Knee Prosthesis “Endo” Model: Surg. Technique
The Journal of Orthopaedic Surgical Techniques, Volume 3,
Number 2, 1987 (K36)
K. Heinert, E. Engelbrecht
Langzeitvergleich der Knie-Endoprothesensysteme St. Georg®
10-Jahres-Überlebensraten von 2236 Schlitten- und Scharnier Endoprothesen
Der Chirurg (1988) 59:755-762 (K38)
F. Madsen, P. Kjarsgaard-Andersen, M. Juhl, O. Sneppen
A Custom-Made Prosthesis for the Treatment of Supracondylar
Femoral Fractures after Total Knee Arthroplasty: Report of Four Cases
Journal of Orthopaedic Trauma, Vol. 3, No. 4, pp. 333-337,1989 (K42)
E. Nieder
Schlittenprothese, Rotationsknie und Scharnierprothese Modell
St. Georg® und Endo-Modell®. Differentialtherapie in der primären
Kniegelenkalloarthroplastik
Orthopäde (1991) 20:170-180 (K45)
G. von Förster, D. Klüber und U. Käbler
Mittel- bis langfristige Ergebnisse nach Behandlung von 118 periprothetischen
Infektionen nach Kniegelenkersatz durch einzeitige Austauschoperationen
Orthopäde(1991) 20: 244-252 (K46)
Adolph V. Lombardi, Jr, Thomas H. Mallory, Robert W. Eberle, and Joanne B. Adams
Results of Revision Total Knee Arthroplasty Using Constrained Prostheses
Seminars in Arthroplasty, Vol 7, No. 4 (October), 1996: pp 349-355
E. Engelbrecht, E. Nieder, D. Klüber
Reconstruction of the Knee - Ten to Twenty Years of Knee Arthroplasty at
the Endo-Klinik: A Report on the Long-term Follow-up of the St. Georg®
Hinge and the Medium-term Follow-up of the Rotating Knee Endo-Model®
Springer Verlag: Tokyo, Berlin, Heidelberg, New York (1997) (K57)
E. Nieder
Revisionsalloarthroplastik des Kniegelenks
Sonderausgabe aus: Orthopädische Operationslehre, Band II/1: Becken und untere Extremität
Herausgegeben von R. Bauer, F. Kerschbaumer und S. Poisel
F. Alt, U. Sonnekalb, N. Walker
Unikondyläre Schlittenprothese versus scharniergeführte
Totalendoprothesen des Kniegelenkes
Orthopädische Praxis 1/98, 34. Jahresgang, Seite 20-24, 1998 (K61)
A. V. Lombardi, T. H. Mallory, R. E. Eberle, J. B. Adams
Rotating Hinge Prosthesis in Revision Total Knee Arthroplasty:
Indications and Results
A Reprint from Surgical Technology International VI, 1998 (K55)
E. Nieder, G.W. Baars, A. Keller
Totaler Tibia-Ersatz Endo-Modell®
Orthopädie Aktuell: Nr. 5/1998, LINK News (K60)
S. Schill, H. Thabe
Die periprothetische Knieinfektion - Therapiekonzept, Wertigkeit
und mittelfristige Ergebnisse
Aktuelle Rheumatologie, Heft 5, 24. Jahrgang, 1999, pp 153-160 (K70)
G.W. Baars
Knieendoprothetik: Das optimale Implantat für jeweilige Indikation finden
Orthopäde 2000 (Suppl1) 29: S1-2
M. Zinck, R, Sellkau
Rotationsknieprothese Endo-Modell®- Geführter Oberflächenersatz mit Sti(e)l
Orthopäde 2000 (Suppl1) 29: S 38-42
M. Crowa, E. Cenna, C. Olivero
Rotating knee prosthesis - Surface or hinge replacement?
Orthopäde 2000 (Suppl1) 29: S 43-44
J-N. Argenson. J M. Aubaniac
Total Knee arthroplasty in femorotibial instability
Orthopäde 2000.29:S 45-47, Springer Verlag 2000 (K72)
M. von Knoch, R. Brocks, C. Siegmüller, G. Ribaric, L. Leupolt,
G. von Förster
Knieflexion nach Rotationsknieendoprothese
Z. Orthop 2000; 138: 66-68 (K71)
R.E. Windsor, K. Steinbrink
Controversies in Total Knee Replacement Two-stage exchange is the optimal treatment for an infected total knee replacement
Oxford University Press 2001 (K78)
A. Katzer, R. Sellckau, W. Siemssen, G. von Foerster
ENDO-Modell Rotating Knee Prosthesis: a functional analysis
J Orthopaed Traumatol (2002) 3:163-170, Springer Verlag 2002
Thomas Nau, MD, E. Pflegerl, MD, J. Erhart, MD, and V. Vecsei, MD
Primary Total Knee Arthroplasty for Periarticular Fractures
The Journal of Arthroplasty, Vol 18, No 8, 2003 (K82)
G. Petrou, H. Petrou, C. Tilkeridis, T. Stavrakis, T. Kapetsis, N.
Kremmidas, M. Gavras
Medium-term results with a primary cemented rotating-hinge total knee replacement
A 7-TO 15-YEAR FOLLOW-UP
J Bone Joint Surg (Br), 2004; 86-B :813-17 (K84)
M.R. Utting, J.H. Newman
Customised hinged knee replacement as a salvage procedure for failed total knee arthroplasty
The Knee 11 (2004) 475-479 (K86)
Nayana Joshi, Antonio Navarro-Quilis
Is There a Place for Rotating-Hinge Arthroplasty in Knee Revision Surgery for Aseptic Loosening?
The Journal of Arthroplasty 2008; 23(8):1204-1210 (K94)
M. Napp, M. Frank, M. Witt
Pathologische Fraktur des distalen Femurs bei Knie-TEP
Der Orthopäde, Band 38, Heft 10, Oktober 2009 (K96)
Dae Kyung Bae, Sang Jun Song, Kyoung Ho Yoon, Jung Ho Noh
Long-Term Outcome of Total Knee Arthroplasty in Charocot Joint:
A 10- to 22- Year Follow-Up
The Journal of Arthroplasty 2009; 24(8):1152-1156 (K98)
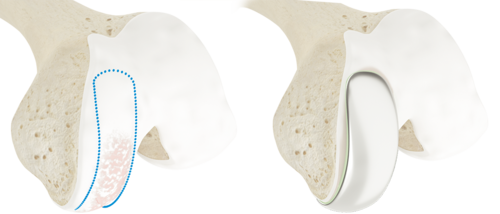
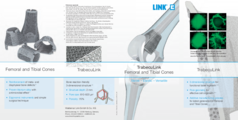
TrabecuLink Femoral and Tibial Cones

Visit also our FlexiCone Microsite - click here!
- Stable – with cementless fixation
- Elastic – due to integral bending axes
- Versatile – for a broad range of solutions 7
The dynamic TrabecuLink Femoral- and Tibial Cones are an attractive solution for cementless restoration of bone defects10 and to provide additional support for the prosthesis if there is bone loss in the proximal tibia. The combination of the dynamic design5,6 of the cones and the biocompatible material Tilastan– E11,12 is ideal for ensuring stable, long-lasting fixation and successful bone regeneration.
The 3-dimensional TrabecuLink structure, with its pore size, porosity and structure depth, also provides an excellent basis for promoting osteoconduction and microvascularization, taking into account the requirements for the structure-covering protein layer (fibronectin - vitronectin - fibrinogen).1,2 TrabecuLink Cones can be used in combination with the long-established LINK Endo-Model knee family in a wide range of sizes and versions. The choice of sizes corresponds to the dimensions of the hinged
knee prostheses.
Stable – in metaphyseal fixation9,13
- Reinforcement of the bone structure in cases of femoral and tibial bone defects
- High primary stability, both for the TrabecuLink Cone itself and for the prosthesis component cemented in the cone
- Cementless interface with the bone for bone regeneration
Elastic – due to integral bending axes in the inner metal wall
- Mechanical compression promotes bone regeneration5,6
- Bending axes for adaptation to bone surfaces
- Good fit ensured by structural elasticity, which also facilitates insertion of the TrabecuLink Femoral/ Tibial Cones
- Spring effect for easier intraoperative positioning
Versatile – for a broad range of solutions7
- Can be combined with all the components of the LINK Endo-Model knee family
- Sizes correspond to the sizes of the hinged knee prostheses
- Customized models can be manufactured
Protective – due to inner metal wall
- Prevents penetration of bone cement into the TrabecuLink structure
- Reliable cement fixation by means of specially positioned “notches” (revision-friendly)
Environmentally friendly3,8
- Resource-saving manufacturing of proven Titanium alloy
TrabecuLink
3-dimensional structure – for optimal bone ongrowth
- Pore geometry (porosity: 70%, pore size: 610-820 μm, structure depth: up to 2 mm) ensures excellent cell ongrowth 1,2,4
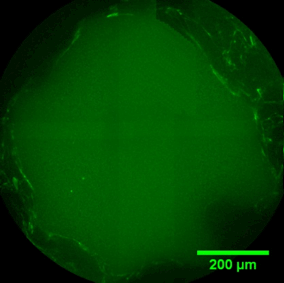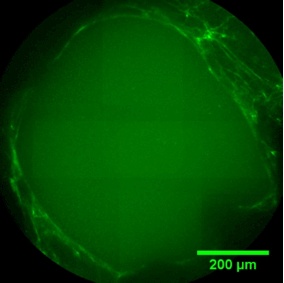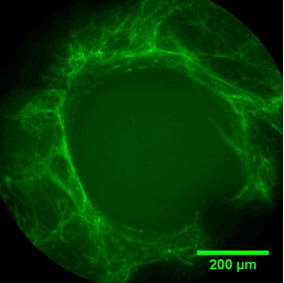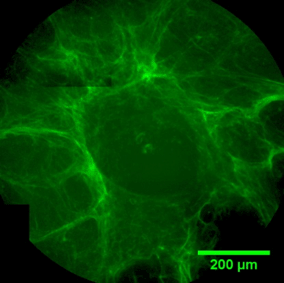
Pore filling
The sequence of images shows a pore of the TrabecuLink structure being filled with tissue under in-vitro cell culture conditions. The fibronectin laid down by human fibroblasts and continually reorganized over a period of eight days is visible as green fibers. Fibronectin is a component of the extracellular matrix that is formed at an early stage of the healing process. It forms a basis for the embedding of collagen, which is essential for mineralization of the tissue and ingrowth of bone into the structure. Apart from the accumulation of fibronectin, which increases over time, a clear contraction of the matrix towards the center of the pore can be observed. This contraction mechanism, which is attributable to the cellular forces acting in the tissue, accelerates the rate at which the pore is filled with tissue, compared to a layer-by-layer tissue growth (Reference: Joly P et al., PLOS One 2013; https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0073545). Julius Wolff Institute, Charité - Universitätsmedizin Berlin
References (general)
- Cecile M. Bidan, Krishna P. Kommareddy, Monika Rumpler, Philip Kollmannsberger, Yves J.M. Brechet, Peter Fratzl, John W.C. Dunlop. et al.; How Linear Tension Converts to Curvature: Geometric Control of Bone Tissue Growth; PLoS ONE 7(5): e36336. https://doi.org/10.1371/journal.pone.0036336 (2012)
- Pascal Joly, Georg N. Duda, Martin Schöne, Petra B. Welzel, Uwe Freudenberg, Carsten Werner, Ansgar Petersen, et al.; Geometry-Driven Cell Organization Determines Tissue Growth in Scaffold Pores: Consequences for Fibronectin Organization; PLoS ONE 8(9): e73545. doi.org/10.1371/journal.pone.0073545 (2013)
- Dr. Malte Drobe, Franziska Killiches; Vorkommen und Produktion mineralischer Rohstoffe – ein Ländervergleich; Bundesanstalt für Geowissenschaften und Rohstoffe Hannover; http://www.bgr.bund.de/DE/Themen/Min_rohstoffe/Downloads/studie_rohstoffwirtschaftliche_einordnung_2014.pdf?__blob=publicationFile&v=4 (2014)
- Steinemann SG; Compatibility of Titanium in Soft and Hard Tissue – The Ultimate is Osseointegration; Materials for Medical Engineering, WILEY-VCH, Volume 2, Page 199-203
- Gerald Küntscher; Praxis der Marknagelung; Friedrich-Karl Schattauer-Verlag (1962)
- R. Texhammer, C. Colton et al.; AO-Instrumente und Implantate (Technisches Handbuch); Springer Verlag, 2. Auflage, S.25 (2011)
- Gabriele Panegrossi, corresponding author Marco Ceretti, Matteo Papalia, Filippo Casella, Fabio Favetti, and Francesco Falez; Bone Loss Management in Total Knee Revision Surgery; Int Orthop. 2014 Feb; 38(2): 419–427; www.ncbi.nlm.nih.gov/pmc/articles/PMC3923937/ (2014)
- Conflict Minerals: MEPs Secure Mandatory Due Diligence for Importers; Press release - External/international trade − 22-11-2016 - 19:07; www.europarl.europa.eu/news/en/news-room/20161122IPR52536/conflict-minerals-meps-secure-mandatory-due-diligence-for-importers (2016)
- Henricson A, Linder L, Nilsson KG.; A Trabecular Metal Tibial Component in Total Knee Replacement in Patients Younger than 60 Years: a Two-year Radiostereophotogrammetric Analysis; J Bone Joint Surg Br. 2008;90:1585–1593. doi: 10.1302/0301-620X.90B12.20797 (2008)
- P. K . Sculco, M. P. Abdel, A. D. Hanssen, D. G. Lewallen; The Management of Bone Loss in Revision Total Knee Arthroplasty; Bone Joint J 2016;98-B(1 Suppl A):120–4 (2016)
- Peter Heinl, Lenka Müller, Carolin Körnera, Robert F. Singera, Frank A. Müllerb; Cellular Ti–6Al–4V Structures with interconnected Macro Porosity for Bone Implants Fabricated by Selective Electron Beam Melting; Acta Biomaterialia Volume 4, Issue 5, September 2008, Pages 1536–1544 (2008)
- Hong Wang, Bingjing Zhao, Changkui Liu, Chao Wang, Xinying Tan, Min Hu; A Comparison of Biocompatibility of a Titanium Alloy Fabricated by Electron Beam; PLOS ONE | DOI:10.1371/journal.pone.0158513 July 8 2016, (2016)
- Ivan De Martino, Vincenzo De Santis, Peter K Sculco, Rocco D’Apolito, Joseph B Assini, Giorgio Gasparini; Tantalum Cones Provide Durable Mid-Term Fixation in Revision TKA; Clin Orthop Relat Res 473 (10), 3176-3182 (2015)