Both, the TrabecuLink Femoral and Tibial Cones, allow a compliant combination with the entire Endo-Model Knee family. In regulatory terms this means an on-label use of this combination.
On the other hand, a combination of our Endo-Model knee family with cones from competitors is an off-label use. In these combinations the surgeon becomes the regulatory manufacturer of this combination. (Please see also abstract from the Direct Link 02-2018 Interview with Mr. Morgan-Jones - Spire Cardiff Hospital, Cardiff)
LINK FlexiCones is the marketing name for our registered TrabecuLink Femoral and Tibial Cones (in short: TL Cones). The trade name is the short form of Flexible Cones.
TrabecuLink is the registered trade name of a 3-dimensional structure and describes the surface of the implant. It is produced in an additive manufacturing process.
3D printing video
https://www.linkorthopaedics.com/de/fuer-den-arzt/flexicones-microsite/video
The pore geometry ensures excellent cell ongrowth.
https://www.linkorthopaedics.com/for-the-physician/products/product-filter?tx_apedvlinkproducts_productfilter%5Baction%5D=show&tx_apedvlinkproducts_productfilter%5Bproduct%5D=115&cHash=6f67bff10012a53800e268bc3eb1af50
They are a class IIb medical device that requires the services of a Notified Body to approve the Declaration of Conformity through a conformity assessment in the CE market.
MEDCERT GmbH in Hamburg
https://www.med-cert.com/notified-body/
TrabecuLink Tibial Cones received CE approval in February 2018.
TrabecuLink Femoral Cones obtained clearance for CE markets in December 2019.
The TrabecuLink Cones were cleared 2020.
OPS 5_785.4h (for metallic bone replacements in the distal femur)
OPS 5_785.4k (for metallic bone replacements in the proximal tibia)
Potential enhanced fixation of hinged knee prostheses by additional zonal fixation and support in the metaphyseal knee area
Dr. Morgan-Jones / Zonal fixation in revision total knee arthroplasty
https://pubmed.ncbi.nlm.nih.gov/25628273/
Dr. Gehrke / Preliminary clinical results of coated porous tibia cones in septic and aseptic revision knee arthroplasty
https://link.springer.com/article/10.1007/s00402-020-03434-2
The purpose of the FlexiCones is to provide support in cases of bone loss, with the objective of stabilization in the distal femur or proximal tibia.
The selected cone is designed to:
- Strengthen the metaphyseal / diaphyseal medullary canal of the distal femur / proximal tibia
- Adequately fill the tibial/femoral bone cavity
- Ensure that assembled components are securely seated, e.g. femoral cone to femoral knee component, while at the same time supporting osteoconduction at the external surface of the cone
- Unique implant design by compensator and spring effect, for an enhanced and safe product positioning with a high initial implant stability
- Special TrabecuLink structure produced in additive manufacturing for functional bone ingrowth
- Performs with the entire Endo-Model knee family for a broad range of solutions
Both, the TrabecuLink Femoral and Tibial Cones, allow a compliant combination with the entire Endo-Model Knee family. In regulatory terms this means an on-label use of this combination.
On the other hand, a combination of our Endo-Model knee family with cones from competitors is an off-label use. In these combinations the surgeon becomes the regulatory manufacturer of this combination. (Please see also abstract from the Direct Link 02-2018 Interview with Mr. Morgan-Jones - Spire Cardiff Hospital, Cardiff)
Both the compensator and the patented bending axes allow the TL Cones to provide flexibility within the cone design. During the development phase, it was found that having built-in flexibility is an advantage. Compared to rigid cones, we found less stress when performing the final seating of the FlexiCones. The introduction of a rigid cone, on the other hand, resulted in bone fissures or even cracks because of an inhomogeneous load transfer.
After implantation, the FlexiCones follow the intramedullary nailing principle: Küntscher's concept of transverse elastic deadlock which can only be achieved by designing the cross-section of the implant itself to be elastic.
The sizes of the FlexiCones conform to the sizing of the well-proven Endo-Model knee family
The FlexiCones and Endo-Model knee prostheses are always compatible 1:1 in terms of sizing.
Further combinations are possible following previous trials.
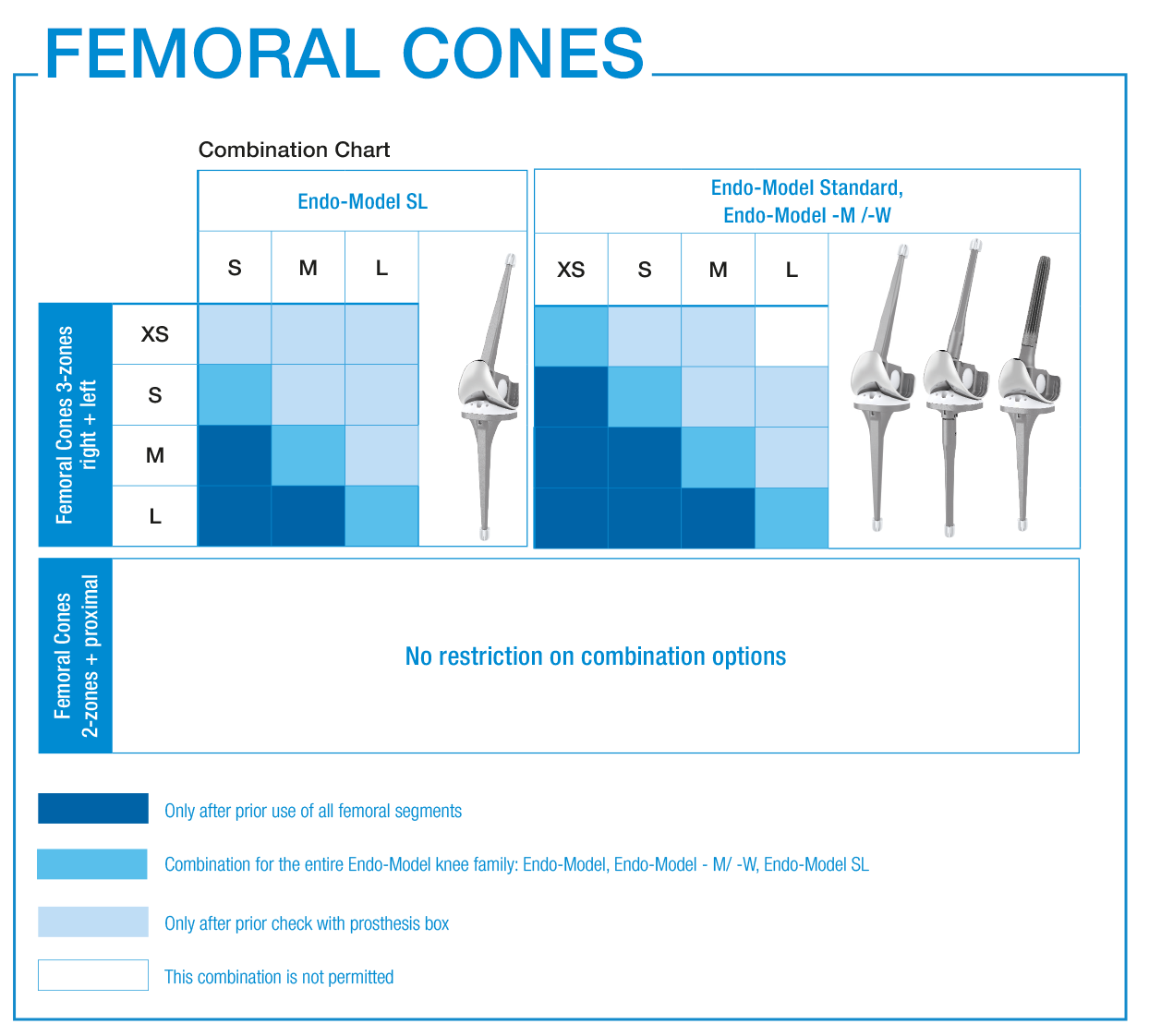
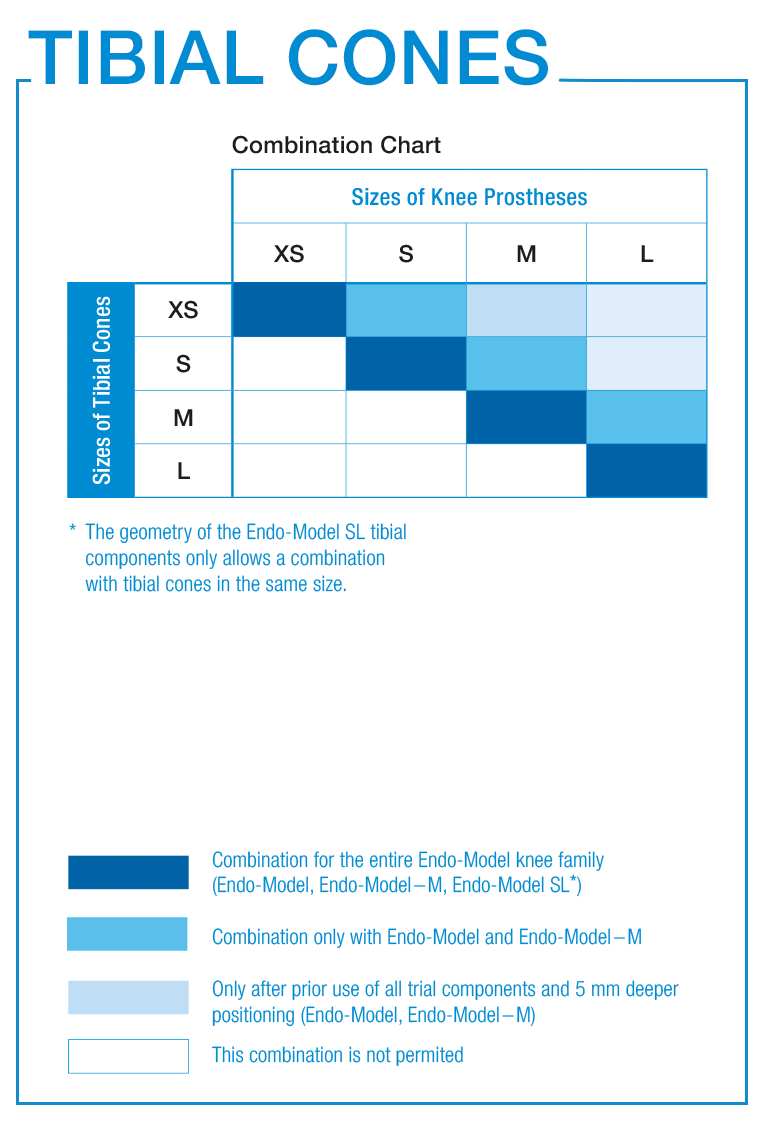
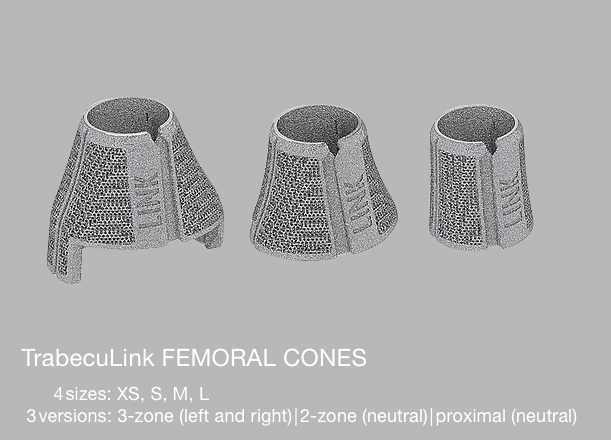
LINK offers 3-zones, 2-zones and proximal TrabecuLink Femoral Cones.
metaphyseal
- The 3-zone Femoral Cones cover the prosthesis box of all Endo-Model femoral components which are to provide additional rotational stability. In accordance with the Endo-Model femoral component, which is available in right/left versions, we also offer the Femoral Cones in right/ left versions.
- The 2-zone Femoral Cones are dimensionally identical, except for the absence of “legs”. Therefore, these Femoral Cones can be implanted side-neutral. Intraoperative positioning offers more freedom as there are no restrictions on the combination used with the Endo-Model femoral components.
diaphyseal
- The proximal Femoral Cones are used in the metaphyseal/diaphyseal area of the femur and are available as a side-neutral version.
Yes.
LINK provides trial cones for the TrabecuLink Tibial Cones. The trial cones are undersized, without built-in elasticity. Only the fins on the outside of the trial cones are identical to the final dimension of the TrabecuLink Tibial Cones. The initial stability results from the increased volume of the final implant and the compression of the cancellous bone during implantation. The press-fit is additionally supported through a built-in spring effect.
The instruments for the TL Femoral Cones combine 2 functions.
The femoral compressors are used for preparation of the implant bed and remain in situ as trial cones. This even helps to compensate for bone shrinkage between trailing and final implantation of the TL Femoral Cone. The dimensions of all TL Cone trials are half of the compressed implant geometry.
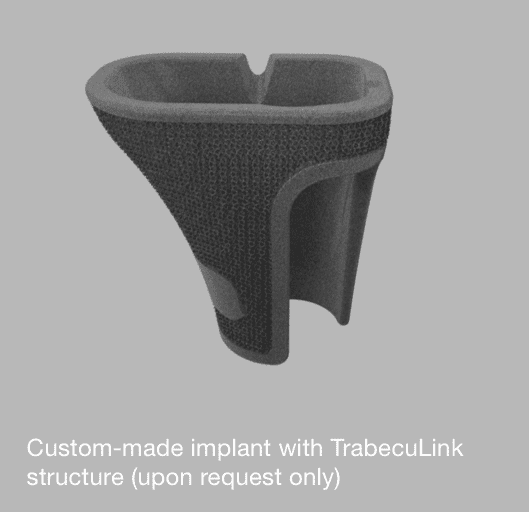
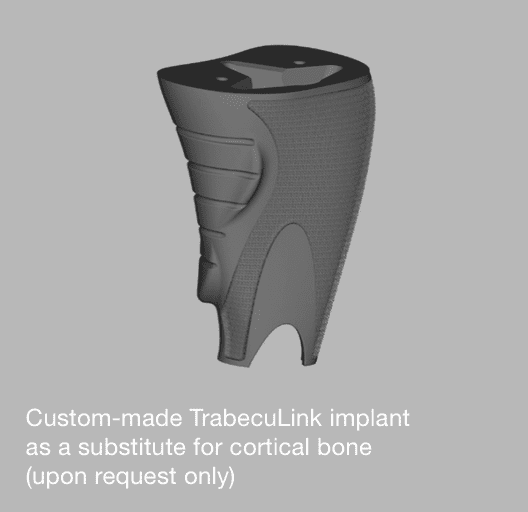
Although our standard sizes cover most cases, there may be special requirements.
For such patients we offer custom-made implants as a solution. Please do not hesitate to contact us with X-rays and/or a CT scan (1mm slides are recommended).
Our experienced CustomLink department will be glad to help you and your patient!
Examples:
LINK recommends always perfoming a test of all trial components, i.e. the trial femoral component, trial stem, trial segment, trial tibial component. After implantation of the Femoral Cone, we recommend double-checking that the FlexiCone is correctly seated and that the stem of the femoral component still fits.
Technically, cementless/ cemented stems with diameters of up to 16 mm (cylindrical) and 18 mm (conical) can be combined.
Follow the standard procedure for cementless modular/ fixed stems and fill the inner void of the implanted FlexiCone shortly before final positioning of the knee component.