SECEC 2025: First Multicenter Clinical Data for the LINK EMBRACE Shoulder System
At this year’s SECEC congress in Rotterdam, The Netherlands, four Spanish centers presented the first multicenter, prospective, one-year outcomes for the LINK EMBRACE Shoulder System
in reverse shoulder arthroplasty (RSA). The analysis provides structured, short-term evidence on safety, effectiveness, implant selection, and complications. It also informs clinical decision-making and post-market evaluation under the European Union Medical Device Regulation (MDR) and is directly relevant to EU Notified Bodies. The LINK EMBRACE Shoulder System is an onlay-based, modular TSA/ RSA platform introduced in 2020, and this is the first poster derived directly from a prospective clinical study of the system.
A Pragmatic Multicenter Snapshot
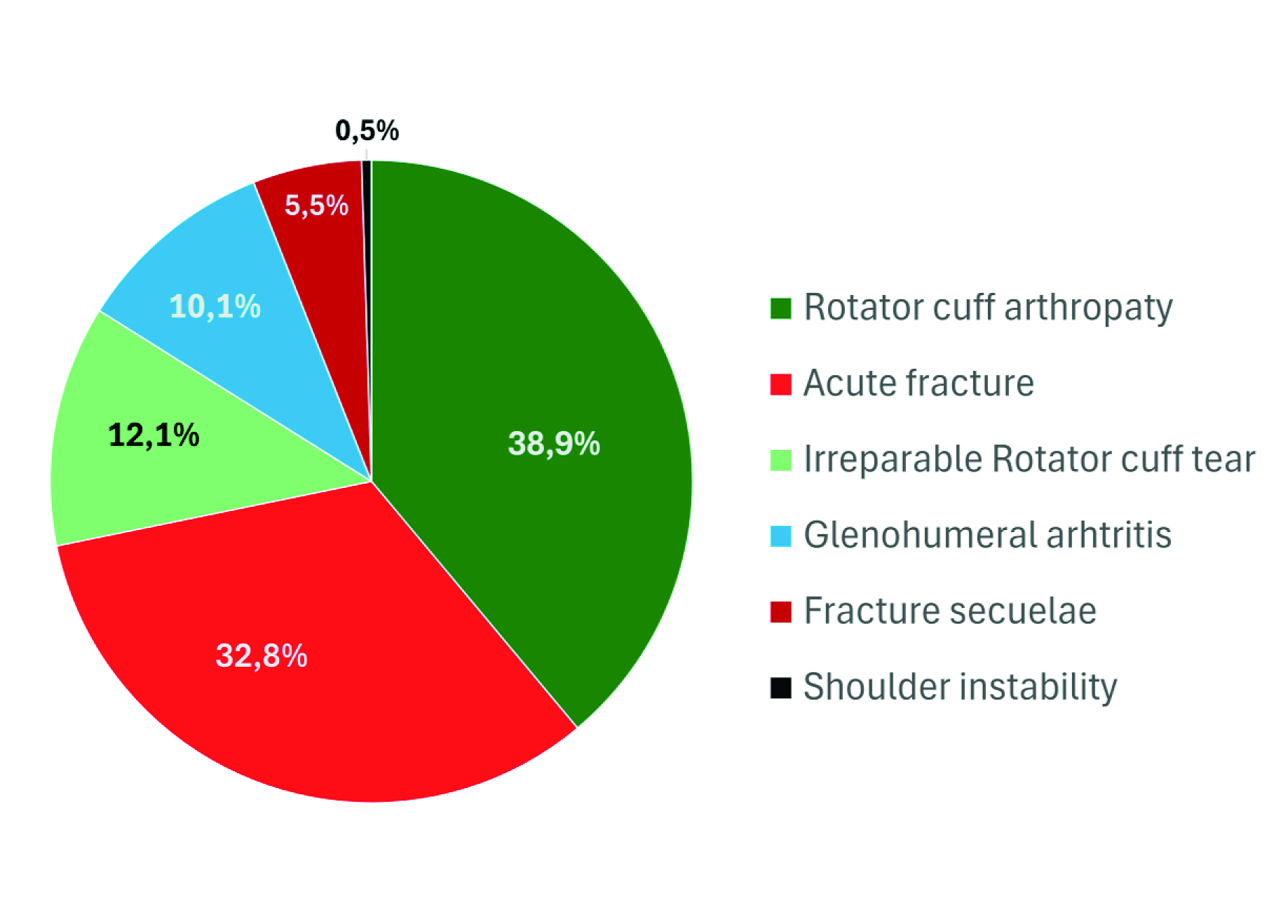
The cohort comprised 219 consecutive primary RSAs, with 198 patients (90.4%) completing the 12-month follow-up. Indications reflected routine practice: cuff-tear arthropathy and acute proximal humerus fractures predominated, followed by irreparable rotator cuff tears, primary osteoarthritis, and fracture sequelae. The program was led by M. Á. Ruiz-Ibán (Madrid), P. Cañete Pastor (Manises/Valencia), E. Melendreras (Murcia), and M. Hermida (Santiago de Compostela). The cohort included 51 men and 147 women; mean age was 72.3 ± 8.02 years. Clinical and radiological data, complications, and reinterventions were recorded intraoperatively and at six weeks and one year follow-up. Across the four centers, the combination of a high 12-month
follow-up rate and a low early revision rate indicates consistent real-world performance in routine practice.
Implant Choices and Techniques in Step with Modern RSA
Humeral fixation was predominantly cementless; modular stems were used in 23.8% of cases based on bone quality and fracture morphology, while stemless use was infrequent. On the
glenoid side, a 28-mm baseplate was standard and most often secured with two screws. Glenospheres were chosen primarily at 39 mm; both concentric and inferior-eccentric options were employed to balance stability, impingement avoidance, and deltoid wrapping. Bony Increased Offset–Reverse Shoulder Arthroplasty (BIO-RSA) was used selectively to lateralize the glenoid with a structural bone graft and mitigate scapular notching while maintaining fixation.
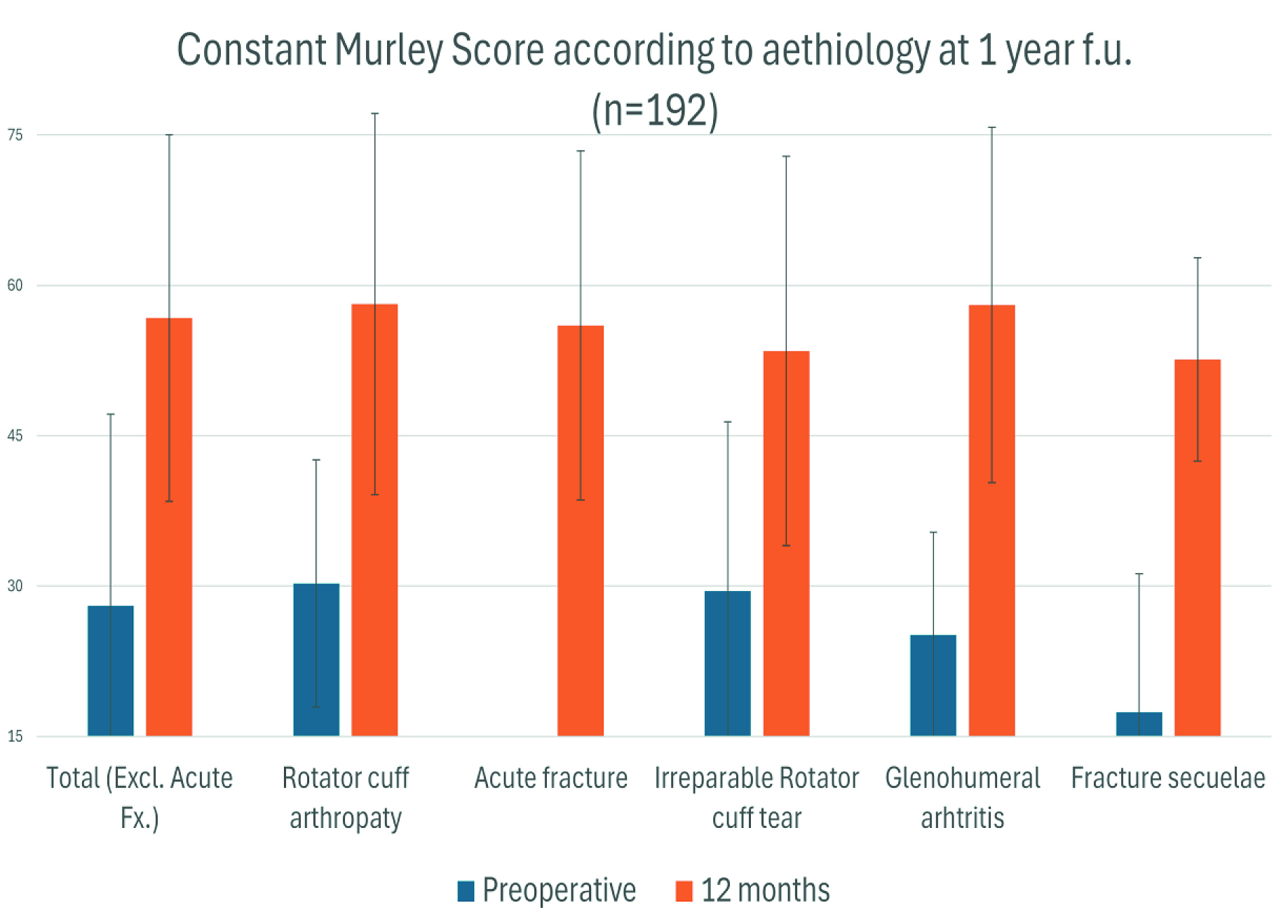
Complications and Revisions
Through 12 months, the overall revision rate was ~3% (6/198). Three patients developed deep infection; two were treated with two-stage revision, and one underwent resection arthroplasty. One patient experienced early stem loosening. One patient sustained a periprosthetic fracture over a loose stem. One patient dislocated and required exchange of the humeral liner. Excluding deep infection failures, the implant-related revision rate was approximately 1.5% (3/198).
Clinical Outcomes at One Year
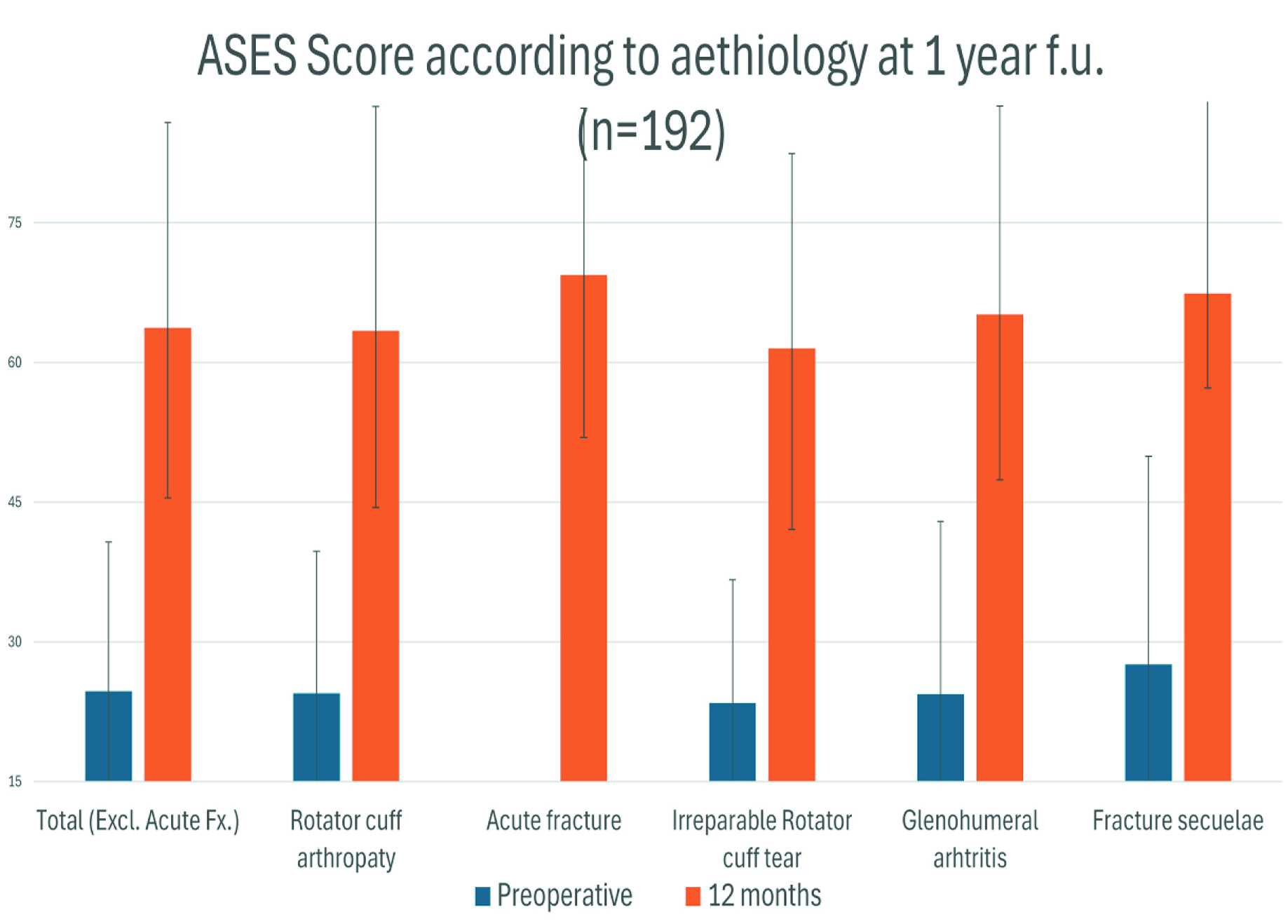
Patients demonstrated statistically significant improvements in both the Constant–Murley score and the American Shoulder and Elbow Surgeons (ASES) score, across indications, indicating functional recovery and symptom relief during the first postoperative year with the LINK EMBRACE platform in routine care.
Interpretation
The prospective, longitudinal, multicenter design with consecutive case capture and a high follow-up rate supports the external validity of these short-term results. The pattern and frequency of complications are consistent with contemporary reverse shoulder arthroplasty in similar populations. Although longer follow-up is necessary to evaluate durability and radiographic remodeling, the one-year data suggest the broad applicability of the LINK EMBRACE Shoulder System for common reverse indications. These one-year data constitute a multicenter milestone, offering an early benchmark for routine EMBRACE use.
Conclusion
Based on the first multicenter, 12-month study dataset, the authors conclude that the LINK EMBRACE Shoulder System is safe and effective in the short term for the management of shoulder conditions that require reverse shoulder arthroplasty. In practical terms, surgeons achieved reliable fixation on both the humeral and glenoid sides; patients exhibited and reported clinically relevant functional improvement within one year; and the early revision rate remained low. Ongoing follow-up will clarify mid-term durability and inform optimization of implant selection across indications.
Context: Evidence and Registries
Beyond investigator-led prospective studies, the manufacturer, LINK, supports real-world evidence generation, including collaboration with the DVSE Shoulder Arthroplasty Registry (German, Austrian and Swiss Society for Shoulder and Elbow Surgery). This registry complements the German Arthroplasty Registry (EPRD) and contributes to clinical evaluation and post-market surveillance under the Medical Device Regulation (MDR). The LINK EMBRACE Shoulder System has now been in clinical use for approximately five years, with results disseminated internationally.
Take-Home Message for Surgical Teams