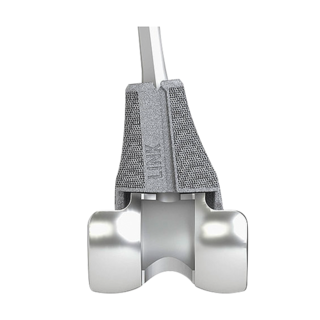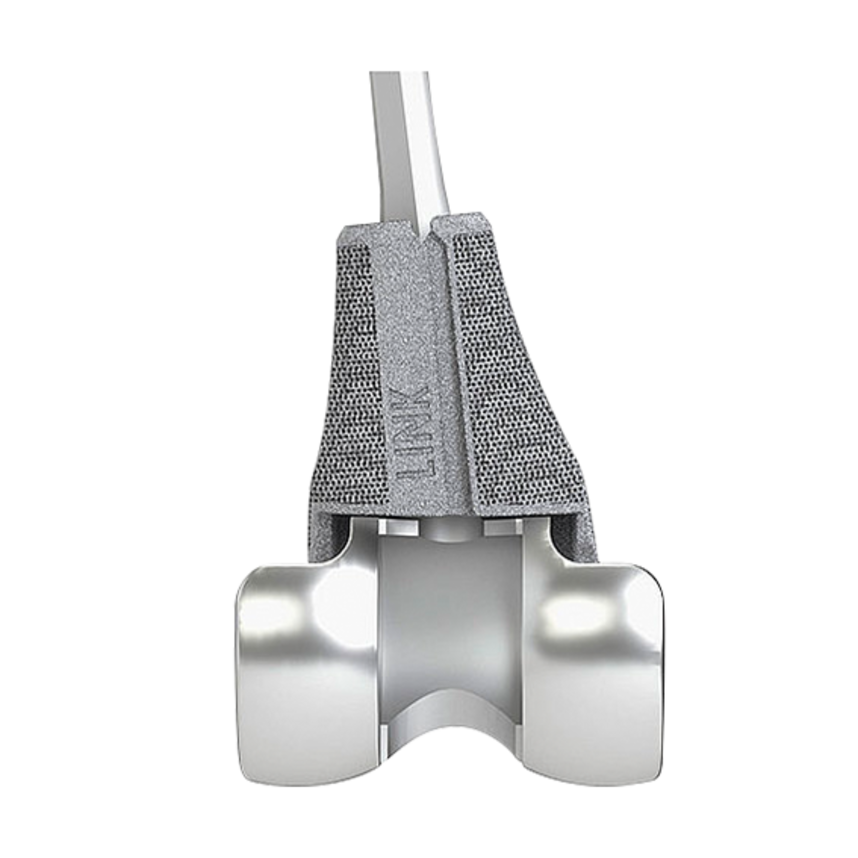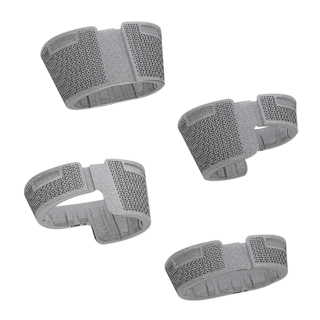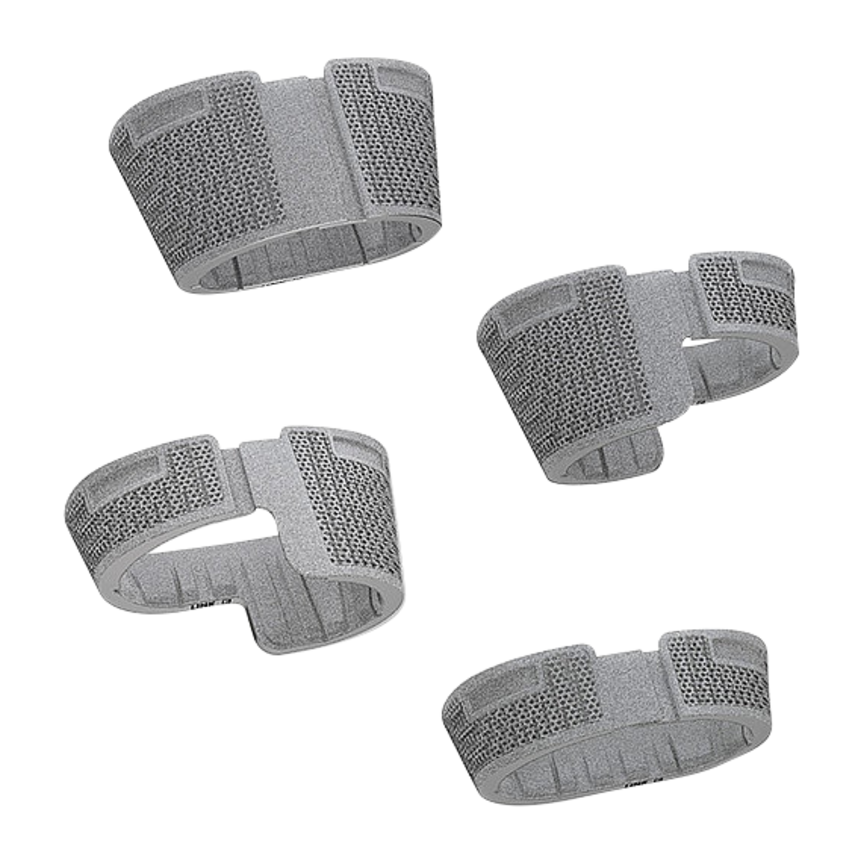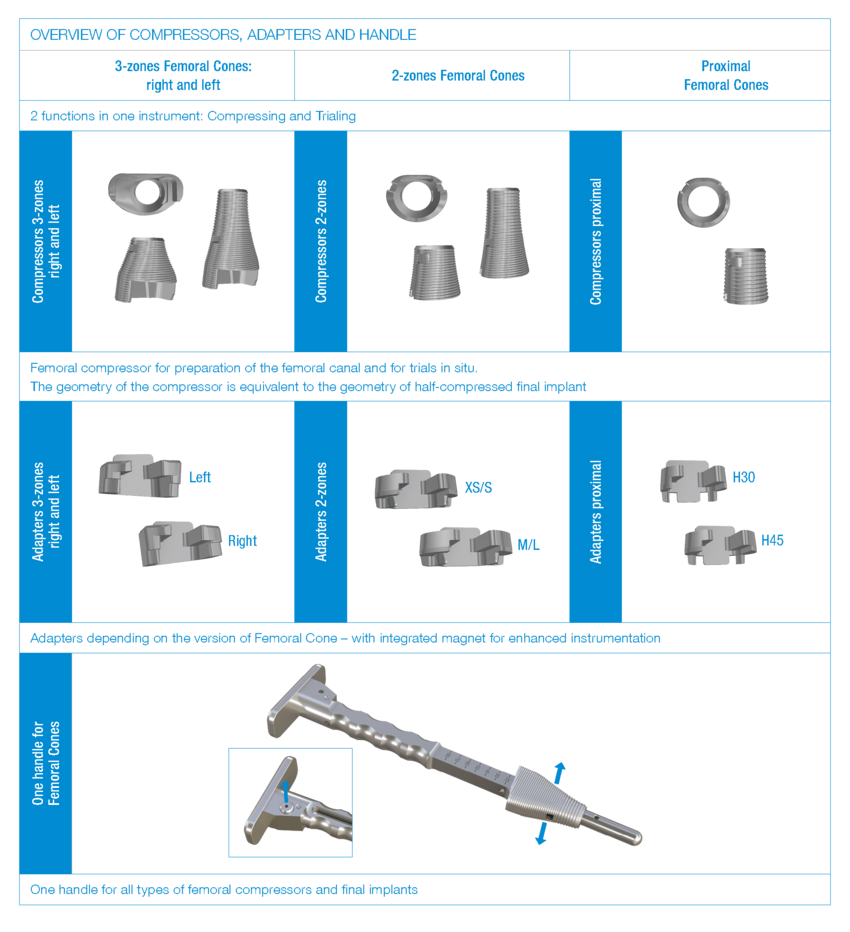
Function of femoral and tibial trial cones
- Slightly undersized, i.e. only the outer ridges are identical to the dimension of the implant
- Without constructive elasticity
- Can be used for compression of bone graft to fill the defects

Example: Trialing and repeated trialing for TrabecuLink Femoral Cones
A trial positioning with trial instruments and a repetition of trialing with a mix of implant and trial knee prosthesis or final implants helps to ensure correct positioning and to confirm the final implant combination, according to the patient’s situation.
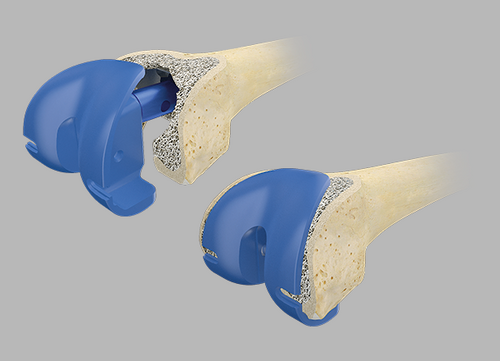
Trial reduction
In order to check that the fit is correct, it is necessary to carry out a trial reduction. In addition to the femoral compressor, which has been placed in the bone, the following trial components from the Endo-Model knee family are required:
- trial femoral component
- trial stem
- segments, if necessary
- trial tibial component
If it is not possible to insert the prosthesis combination correctly, further preparation must be carried out as described above.

Repeat trial
After implantation of the Femoral Cone, it is advisable to double-check that it is correctly seated before placing the bone cement. This can be done with either the fully assembled femoral component (in the
case of modular implant systems) or the femoral trial component. The purpose of the repeat trial, especially when using the 3-zone Femoral Cones is to provide an intraoperative check for the appropriate box level between Femoral Cone and femoral knee component.

STABLE – IN METAPHYSEAL FIXATION9,13
- Reinforcement of the bone structure in cases of femoral and tibial bone defects
- High primary stability, both for the TrabecuLink Cone itself and for the prosthesis
component cemented in the cone - Cementless interface to the bone for biologic fixation
ELASTIC – DUE TO INTEGRAL BENDING AXES IN THE INNER METAL WALL
- Mechanical compression of the bone promotes bone remodeling5,6
- Bending axes for adaptation to bone surfaces
- Good fit ensured by structural elasticity, which also facilitates insertion of the TrabecuLink Femoral/Tibial Cones
- Spring effect for easier intraoperative positioning
VERSATILE – FOR A BROAD RANGE OF SOLUTIONS7
- Can be combined with all components of the LINK Endo-Model knee family
- Sizes correspond to the dimensions of the hinged knee prostheses
PROTECTIVE – DUE TO INNER METAL WALL
- Prevents penetration of bone cement into the TrabecuLink structure
- Reliable cement fixation by means of specially positioned “notches” (revision-friendly)
ENVIRONMENTALLY FRIENDLY3, 8
- Resource-saving manufacturing with proven titanium alloy
The dynamic TrabecuLink Femoral and Tibial Cones are an attractive solution for the cementless restoration of bone defects10 and for providing additional support to the prosthesis if there is bone loss in the proximal tibia or distal femur. The combination of the dynamic design5,6 of the cones, the biocompatible material Tilastan– E11,12, and the 3-dimensional TrabecuLink structure is designed to provide is stable, long-lasting fixation.
TrabecuLink Cones can be used in combination with the long-established LINK Endo-Model knee family in a wide range of sizes and versions. The choice of sizes corresponds to the dimensions of the hinged knee prostheses.
The 3-dimensional structure is produced in additive manufacturing process.
The optimized pore geometry for effective cell ongrowth for intended second stability.
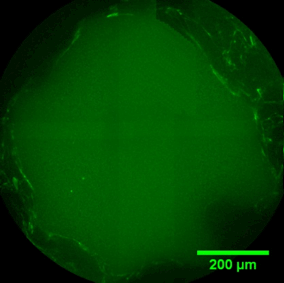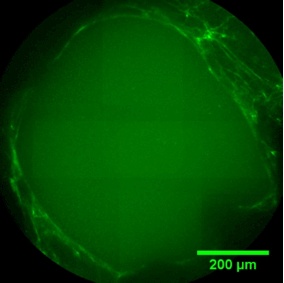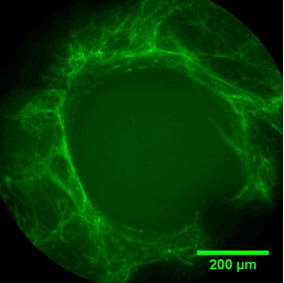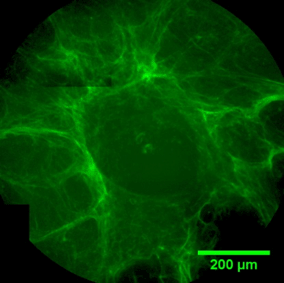
PORE FILLING
The sequence of images shows a pore of the TrabecuLink structure being filled with tissue under in-vitro cell culture conditions. The fibronectin laid down by human fibroblasts and continually reorganized over a period of eight days is visible as green fibers. Fibronectin is a component of the extracellular matrix that is formed at an early stage of the healing process. It forms a basis for the embedding of collagen, which is essential for mineralization of the tissue and ingrowth of bone into the structure. Apart from the accumulation of fibronectin, which increases over time, a clear contraction of the matrix towards the center of the pore can be observed. This contraction mechanism, which is attributable to the cellular forces acting in the tissue, accelerates the rate at which the pore is filled with tissue, compared to a layer-by-layer tissue growth.*
* In-vitro testing may not be indicative of clinical results.
References (general)
- Cecile M. Bidan, Krishna P. Kommareddy, Monika Rumpler, Philip Kollmannsberger, Yves J.M. Brechet, Peter Fratzl, John W.C. Dunlop. et al.; How Linear Tension Converts to Curvature: Geometric Control of Bone Tissue Growth; PLoS ONE 7(5): e36336. https://doi.org/10.1371/journal.pone.0036336 (2012)
- Pascal Joly, Georg N. Duda, Martin Schöne, Petra B. Welzel, Uwe Freudenberg, Carsten Werner, Ansgar Petersen, et al.; Geometry-Driven Cell Organization Determines Tissue Growth in Scaffold Pores: Consequences for Fibronectin Organization; PLoS ONE 8(9): e73545. https://doi.org/10.1371/journal.pone.0073545 (2013)
- Dr. Malte Drobe, Franziska Killiches; Vorkommen und Produktion mineralischer Rohstoffe – ein Ländervergleich; Bundesanstalt für Geowissenschaften und Rohstoffe Hannover; http://www.bgr.bund.de/DE/Themen/Min_rohstoffe/Downloads/studie_rohstoffwirtschaftliche_einordnung_2014.pdf?__blob=publicationFile&v=4 (2014)
- Steinemann SG; Compatibility of Titanium in Soft and Hard Tissue – The Ultimate is Osseointegration; Materials for Medical Engineering, WILEY-VCH, Volume 2, Page 199-203
- Gerald Küntscher; Praxis der Marknagelung; Friedrich-Karl Schattauer-Verlag (1962)
- R. Texhammer, C. Colton et al.; AO-Instrumente und Implantate (Technisches Handbuch); Springer Verlag, 2. Auflage, S.25 (2011)
- Gabriele Panegrossi, corresponding author Marco Ceretti, Matteo Papalia, Filippo Casella, Fabio Favetti, and Francesco Falez; Bone Loss Management in Total Knee Revision Surgery; Int Orthop. 2014 Feb; 38(2): 419–427; www.ncbi.nlm.nih.gov/pmc/articles/PMC3923937/ (2014)
- Conflict Minerals: MEPs Secure Mandatory Due Diligence for Importers; Press release - External/international trade − 22-11-2016 - 19:07; www.europarl.europa.eu/news/en/news-room/20161122IPR52536/conflict-minerals-meps-secure-mandatory-due-diligence-for-importers (2016)
- Henricson A, Linder L, Nilsson KG.; A Trabecular Metal Tibial Component in Total Knee Replacement in Patients Younger than 60 Years: a Two-year Radiostereophotogrammetric Analysis; J Bone Joint Surg Br. 2008;90:1585–1593. doi: 10.1302/0301-620X.90B12.20797 (2008)
- P. K . Sculco, M. P. Abdel, A. D. Hanssen, D. G. Lewallen; The Management of Bone Loss in Revision Total Knee Arthroplasty; Bone Joint J 2016;98-B(1 Suppl A):120–4 (2016)
- Peter Heinl, Lenka Müller, Carolin Körnera, Robert F. Singera, Frank A. Müllerb; Cellular Ti–6Al–4V Structures with interconnected Macro Porosity for Bone Implants Fabricated by Selective Electron Beam Melting; Acta Biomaterialia Volume 4, Issue 5, September 2008, Pages 1536–1544 (2008)
- Hong Wang, Bingjing Zhao, Changkui Liu, Chao Wang, Xinying Tan, Min Hu; A Comparison of Biocompatibility of a Titanium Alloy Fabricated by Electron Beam; PLOS ONE | DOI:10.1371/journal.pone.0158513 July 8 2016, (2016)
- Ivan De Martino, Vincenzo De Santis, Peter K Sculco, Rocco D’Apolito, Joseph B Assini, Giorgio Gasparini; Tantalum Cones Provide Durable Mid-Term Fixation in Revision TKA; Clin Orthop Relat Res 473 (10), 3176-3182 (2015)