MobileLink


Many years of experience with successful implant systems and a range of fixation concepts, together with state-of-the-art material and coating technologies, have gone into the development of this new acetabular cup system. The result is the versatile cementless MobileLink Acetabular Cup System.
The MobileLink Acetabular Cup System comes in two different versions: A cluster-hole press-fit cup and a multi-hole press-fit cup. Both versions of the shells are available with a PlasmaLink coating or with a TiCaP double coating.
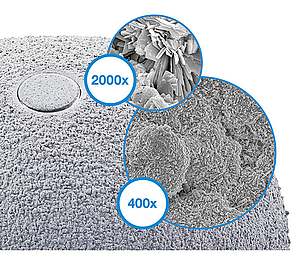
The TiCaP double coating combines a highly porous surface to achieve primary fixation and a calcium phosphate coating, which together ensure optimal primary and secondary implant stability.
The MobileLink Acetabular Cup System can be used with UHMWPE inserts. UHMWPE inserts are available in Standard UHMWPE and E-DUR (X-LINKed, Vit-E PE) versions. All UHMWPE inserts are available in a standard version and also with protection against luxation. The MobileLink Acetabular Cup System can be combined with modular offset and/or inclining casing/insert adapters (Face Changer). The adapters allow restoration of the anatomy in revision cases.

The MobileLink Acetabular Cup System can be transformed into a modular dual mobility system, with the use of Dual Mobility Inserts made from EndoDur. The DM Insert is to accommodate poly DM Liners from the BiMobile Dual Mobility System.
The dual mobility concept was developed by Prof. Gilles Bousquet in the 1970s with the aim of avoiding recurrent hip luxations. A modular Dual Mobility System is composed of a Dual Mobility Insert with a highly polished inner surface placed in a Shell in which a mobile polyethylene Liner with a pressed-in Prosthesis Head is moving.
Features and Advantages of the Dual Mobility System:
- Dual mobility leads to reduced risk of dislocation and increased range of motion (RoM) 4
- Polished inner surface for minimized wear and a prolonged implant life 5,6
- Self-centering Liner promotes even wear patterns and enhances dislocation resistance 7

Features and advantages
Wide choice of sizes (Ø 42-80 mm)
Choice of latest materials, such as E-DUR Polyethylene
Clinically proven rough TiCaP double coating2
Secure – triple fixated inserts
Unique technology of the Face Changer fixation1,3
50/36 mm – small outside, large inside
Color coding for efficient work flow
High flexibility, minimal stock holding
Intraoperative flexibility
Simple instrument set and color coding for efficient surgical procedures
Casing/insert adapter (Face Changer) for anatomical reconstruction1
Variable options for placing bone screws 1
- Data on File, Waldemar Link.
- Ullmark G, Sorensen J, Nilsson O. Analysis of bone formation on porous and calcium phosphate-coated acetabular cups: a randomised clinical [18F]fluoride PET study. Hip international: the journal of clinical and experimental research on hip pathology and therapy. 2012;22(2):172-8.
- PCT-Patent Application WO 2017/140497 A1
- Stroh, D. Alex, et al. "Dual-mobility bearings: a review of the literature." Expert review of medical devices 9.1 (2012): 23-31.
- Long, M., & Rack, H. (1998). Titanium alloys in total joint replacement—a materials science perspective. Biomaterials, 19(18), 1621-1639
- Loving L, Herrera L, Banerjee S, Heffernan C, Nevelos J, Markel DC, Mont MA. 2015. Dual mobility beari ngs withstand loading from steeper cup-inclinations without substantial wear. J Orthop Res. 33(3):398-404.
- Fabry C, Kaehler M, Hermann S, Woernle C, Bader R. 2014. Dynamic behavior of tripolar hip endoprostheses under physiological conditions and their effect on stability. Medical Engineering & Physics 36:65– 71.